The Toxicities of Allogeneic Transplantation: What Fellows Need to Know


Articles in Hematopoiesis are written for trainees by trainees, under the oversight of the ASH Trainee Council. The material published in Hematopoiesis is for informational purposes only. The opinions of the authors are their own and do not necessarily represent the official policy of the American Society of Hematology. ASH does not recommend or endorse any specific tests, physicians, products, procedures, or opinions, and disclaims any representation, warranty, or guarantee as to the same. Reliance on the information provided in this publication is solely at your risk.
 Spoiler alert! (Well, not really a spoiler…) If you haven’t read part one of this two-part series on what fellows need to know about allogeneic transplantation, read it before continuing.
Spoiler alert! (Well, not really a spoiler…) If you haven’t read part one of this two-part series on what fellows need to know about allogeneic transplantation, read it before continuing.
Allogeneic hematopoietic stem cell transplantation (HSCT) is one of the most complex therapies we offer as hematologists, and understanding its intricacies takes decades of practice. We’ve focused on introducing these types of important topics in our “What Fellows Need to Know” series for Hematopoiesis, with previous articles covering measurable residual disease, chimeric antigen receptor T-cell (CAR-T) therapy, and indications for transplantation. Our most recent installment discussed the basics of transplantation itself: in brief, why we use HSCT and where transplanted cells come from. In this article, we turn a closer eye to selected toxicities of allogeneic HSCT that you should be aware of as a trainee.
Toxicities of allogeneic HSCT are typically split into two groups: infectious and non-infectious.
Table 1. Key complications of allogeneic HSCT
| Infectious Complications | Non-Infectious Complications |
| Gram negative bacteria (e.g., Escherichia coli, Pseudomonas aeruginosa, etc.) | Acute GVHD (skin, gastrointestinal [GI] tract, liver) |
| Gram positive bacteria (e.g., MRSA, Streptococcus pneumoniae, etc.) | Chronic GVHD (skin, liver, lung, ocular, oral, vulvovaginal, autoimmune, etc.) |
| Pneumocystis jirovecii | VOD/SOS (e.g., liver, pulmonary) |
| Toxoplasma gondii | Oral Mucositis |
| CLABSI | Idiopathic Pneumonia Syndrome |
| Mycobacterial Infections | Diffuse Alveolar Hemorrhage |
| Clostridium difficile | CRS |
| Invasive fungal infections (e.g., Aspergillus, Mucormycetes, Candida, etc.) | Thrombotic microangiopathy (both complement- and non-complement-mediated) |
| Adenoviruses | Engraftment Syndromes |
| Respiratory Viruses (e.g., Influenza, Parainfluenza, Coronaviruses, etc.) | Graft Failure |
| Enteroviruses (e.g., Rotavirus, Norovirus, etc.) | Secondary Malignancies |
| Herpesviruses (e.g., CMV, EBV [including PTLD], VZV, HHV-6, etc.) | Cardiac Arrhythmias (e.g., Atrial Fibrillation) |
| Polyomaviruses (e,g,, BK virus, JC virus) | Sexual Dysfunction & Infertility |
Abbreviations: HSCT, hematopoietic stem cell transplantation; MRSA, methicillin-resistant Staphylococcus aureus; CMV, Cytomegalovirus; EBV, Epstein-Barr virus; PTLD, post-transplant lymphoproliferative disorder; VZV, varicella-zoster virus; HHV, human herpesvirus; GVHD, graft-versus-host disease; CLABSI, central line-associated bloodstream infection; VOD/SOS, vaso-occlusive disease/sinusoidal obstruction syndrome; CRS, cytokine release syndrome.
As you can see, the list is quite long. We will focus on the first two non-infectious complications because they are must-know entities for trainees: graft-versus-host disease (GVHD) and veno-occlusive disease/sinusoidal occlusive syndrome (VOD/SOS). We have chosen these two given their vague names yet specific manifestations. For some of the other toxicities, the American Society for Transplantation and Cellular Therapy (ASTCT) has released specific guidelines: Gram-negative bacteria,1 invasive fungal infections,2 cytomegalovirus (CMV) prevention,3 and CMV treatment.4 The Hematology, ASH Educational Program has also covered important non-infectious complications including cardiopulmonary toxicities,5 financial toxicity,6 and late effects.7 Some other key transplant-specific complications to be aware of include idiopathic pneumonia syndrome8 (which responds rapidly to early treatment with etanercept9), diffuse alveolar hemorrhage, and thrombotic microangiopathy. If you’d like a dedicated “What Fellows Need to Know” installment for these or any other topics, let us know. Let’s dive in!
GVHD
Now, let’s pivot to GVHD. As we noted in our first installment about HSCT, GVHD and graft-versus-tumor (GVT) are two sides of the same coin. We want a patient’s new immune system to be savvy enough to detect tumor cells as foreign and destroy them. When the patient’s new immune system also attacks bystander host cells after deeming them foreign, GVHD is the result. Unfortunately, GVHD is one of the most common toxicities of HSCT. While estimates vary immensely between studies, it’s fair to estimate that approximately half of HSCT recipients will develop acute and/or chronic GVHD.10
One point of clarification before we continue: the “opposite” of GVHD, in a sense, is graft failure which represents the equivalent of host-versus-graft disease. In graft failure,11 the host’s immune system either prevents the donor stem cells from ever engrafting, resulting in a failure to achieve an absolute neutrophil count of at least 500/μL (primary graft failure), or subsequently evicts the donor cells after initial engraftment requiring growth factor or transfusion support (secondary graft failure). Graft failures represent a spectrum of immune- and non-immune-mediated causes, including suboptimal doses or quality donor cells. Graft rejection is specifically immune-mediated graft failure. Graft failure often manifests with cytopenias and decreasing donor chimerism (percent of cells that belong to the donor12). Since we anticipate initial count recovery driven by mature progenitor cells13 to occur at approximately 17 to 20 days after transplantation, graft failure cannot be diagnosed until at least day +30 after peripheral blood stem cell transplantation or bone marrow transplantation (or day +42 after umbilical cord transplantation). Incidence of graft failure is approximately 5 percent,14 but this is heavily influenced by transplant-related factors such as underlying disease (e.g., nonmalignant disease or myelofibrosis), donor type (e.g., mismatched or haploidentical donors), graft type and dose (e.g., marrow), and conditioning intensity (e.g., reduced-intensity). Graft failure is a true transplant emergency that almost certainly ends in a second transplant or death.
What are the differences between acute GVHD versus chronic GVHD?
Acute GVHD is mainly mediated by alloreactive T cells and, as the name suggests, occurs more acutely after HSCT (generally within the first 100 days). Chronic GVHD is mainly mediated by alloreactive T and B cells and occurs months to years later. There is of course overlap between the two, and acute GVHD is one of the biggest risk factors for chronic GVHD.
Acute GVHD affects three organs: skin (rashes), liver (elevated transaminases), and the gastrointestinal (GI) tract (nausea/vomiting and/or diarrhea). Chronic GVHD on the other hand can affect many organs but often is noted in the skin (tighter skin with decreased mobility), joints (difficulty with ambulation), or eyes and mouth (sicca-like syndrome with dry eyes and/or dry mouth). Among the most severe complications of chronic GVHD is pulmonary GVHD, termed bronchiolitis obliterans syndrome, which can become severely debilitating or fatal.
One point to keep in mind is that GVHD can extend to every organ with non-classical manifestations. Theoretically, any conceivable autoimmune disease can occur after HSCT, whereby the definition of everything is alloimmune. As such, “it’s GVHD until proven otherwise” is a mantra you may often hear in clinics when caring for long-term survivors of HSCT.
How is GVHD diagnosed? And how is it staged, graded, and scored?
Even with a plethora of investigational biomarkers and tools, GVHD remains a clinical diagnosis in the year 2022. There isn’t a specific test that can rule GVHD in or out – even endoscopic biopsies for GI GVHD can miss about a quarter of cases.15 Thankfully, a substantial amount of collaborative effort over the past few decades has gone into standardizing how GVHD is diagnosed and assessed. Here are the key terms established:
- Staging of acute GVHD: Organ-specific toxicities (skin, liver, upper GI, and lower GI) are staged from 0 to 4 using MAGIC criteria.16 For example, skin involvement is staged from 1 to 4 based on the involved percentage of body surface area. Solid malignancies are similarly staged based on degree of spread.
- Grading of acute GVHD: Based on the MAGIC criteria,16 specific combinations of organ-specific staging are combined into a single gestalt grade with Roman numerals. For example, mild skin involvement alone is Grade I GVHD; however, any liver or GI involvement makes acute GVHD grade II or higher. This is easy to remember because cumulative toxicities of chemotherapy (think CTCAE) are graded using a single number.
- Scoring of chronic GVHD: Based on National Institutes of Health criteria,17 chronic GVHD involvement of each organ is scored from 0 to 3. It’s worth reviewing these criteria to see the breadth of possibly involved organs. While we often focus on skin/joint and mucosal manifestations as typical signs of chronic GVHD, any organ can be affected.
What are some risk factors or ameliorating risk factors for GVHD?
This is a complex yet heavily simplified topic that we summarized in Table 2. Remember that GVHD risk is not the only consideration factored into allogeneic HSCT.
The strategies listed in the “higher GVHD risk” are often used to maximize GVT effect or because of donor-/host-related considerations.
Table 2. Risk factors and ameliorating risk factors for GVHD
| Lower GVHD risk | Higher GVHD risk | |
| Donor type | Sibling match (genotypically identical for all HLA antigens, not just the 10-12 we check for) or matched unrelated donor (identical for all 10-12 antigens that we check for) | Mismatched donor (i.e., at least one known mismatched HLA antigen) or haploidentical donor (i.e., parent or child with one shared set of chromosomes). |
| Cells in bag | More CD34+ stem cells and fewer T cells. Think of a graft extracted directly from bone marrow or strategies to artificially alter this balance (ATG, T-cell depletion, CD34+ selection). | Fewer CD34+ stem cells and more T cells. Think of a graft extracted from a donor’s peripheral blood, where more mature cells can sneak in. |
| Pre-HSCT chemo | Less chemo means less tissue damage and less GVHD. In general, RIC or NMA regimens carry a lower risk of GVHD. | More chemo means more tissue damage and more GVHD. Myeloablative regimens thus have a higher risk of GVHD in general. |
| Other donor factors | Umbilical cord blood generally carries a lower risk of GVHD. | Certain donor characteristics (e.g., female donor to male recipient) might increase the risk of GVHD. |
Abbreviations: ATG, anti-thymocyte globulin; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; NMA, non-myeloablative conditioning; RIC, reduced-intensity conditioning.
How do we prevent GVHD pharmacologically?
This is a great question with multiple “it depends” answers, which vary between countries and institutions. There are several classes of medications used for GVHD prophylaxis with a variety of mechanisms of action.
Table 3. Drug classes and mechanisms for GVHD prophylaxis
| Class | Drugs | Mechanism of Action |
| Antigen T-cell receptor interaction |
Anti-thymocyte globulin, alemtuzumab |
Depletion of T lymphocytes |
| T-cell receptor signal transduction |
Cyclosporine, tacrolimus |
Calcineurin blockade |
| IL-2 upregulation |
Basiliximab, daclizumab, denileukin diftitox |
IL-2 receptor blockade, IL-2–directed toxin |
| IL-2 signaling | Sirolimus | mTOR blockade |
| Cell cycle progression |
Mycophenolate mofetil, methotrexate, pentostatin |
DNA synthesis blockade |
Historically, most centers have used a combination of two to three drugs from the following classes:
- Calcineurin inhibitors: Tacrolimus or cyclosporine (though the latter has fallen out of favor in the United States)
- mTOR inhibitors: Primarily sirolimus.
- Anti-metabolites: For example, cyclophosphamide, mycophenolate, or methotrexate within the first month following HSCT.
- Anti-thymocyte globulin (ATG): See Table 3. ATG has lost a bit of its glamour in HSCT from matched unrelated donors because of a phase III trial18 demonstrating increased mortality, but other studies have indicated equivalent survival with decreased GVHD. It remains a standard of care in Europe19 because of positive trials.
In the past two decades, post-transplantation cyclophosphamide (PTCy) has rapidly gained traction. PTCy is given on days +3 and +4 to selectively deplete allo-reactive donor T cells. The conceptual beauty of PTCy is that instead of depleting all donor T cells, it will only deplete T cells that are actively dividing on days +3 and +4. What donor T cells would be so active so quickly after transplantation? The angry allo-reactive “passenger” ones that have already recognized an innocent host protein as foreign and are en route to causing GVHD.
This approach, however, only works if donor T cells are not inhibited until after PTCy. As such, other immunosuppressants like tacrolimus are generally started only after PTCy is used. Additionally, PTCy has been studied most extensively in haploidentical HSCT based on seminal work at Johns Hopkins. Whether PTCy is safe and effective with other donor types is an area of ongoing investigation.
Lastly, we’ll touch on a newly U.S. Food and Drug Administration (FDA)-approved medication for GVHD prevention in certain cases. Abatacept is a CTLA4 agonist that inhibits T-cell activation and proliferation in response to antigen binding (for those of you taking your onc boards, abatacept is essentially the opposite of the checkpoint inhibitor ipilimumab). This approval happened in December 2021,20 so expect more data about this drug in the coming years.
How do we treat acute GVHD?
- Try to minimize systemic corticosteroid exposure: For skin-only GVHD, for example, consider topical steroids. For mild GI-only GVHD, consider oral budesonide or beclomethasone (which has poor bioavailability).
- For GI GVHD, stool output is imperative: Yes, this means quantitating stool output, as well as hospitalizing patients for Grade 2+ or higher GI GVHD (>500 mL per day) and making them NPO. When reintroducing enteral feeding, the mantra is to start low and go slow. Consider additional causes of diarrhea (e.g., infections) and have a low threshold to use total parenteral nutrition as you initiate GVHD-directed therapy.
- When systemic corticosteroids are used, use 1 to 2 mg/kg and taper slowly: Responses generally occur within the first week if they are going to happen, at which point steroids can be tapered slowly (over weeks to months, not days). Steroid-refractory GVHD is generally defined as non-response within five to seven days of therapy, or alternatively as progression to a higher stage within three to five days of therapy.
- Sirolimus is a reasonable alternative to steroids for standard-risk acute GVHD:21 An open-label, randomized phase II trial comparing the two demonstrated similar outcomes with reduced hyperglycemia and infections and improved immune suppression discontinuation and patient-reported quality of life, but increased risk for thrombotic microangiopathy.
- Ruxolitinib is our new standard of care for corticosteroid-refractory acute GVHD: Performing randomized trials in GVHD is notoriously difficult, and so a shout-out to the randomized phase III REACH-2 trial22 is warranted. In this study, ruxolitinib outperformed the investigators’ choices of therapy in terms of response rates and durations. Of note, rapid discontinuation of ruxolitinib (whether for myelofibrosis or for GVHD) can lead to rebound symptoms, so also taper ruxolitinib slowly.
How do we treat chronic GVHD?
A helpful example of a sample algorithm to treat chronic GVHD is shown below (slide courtesy of Dr. Aaron Logan, MD, PhD):
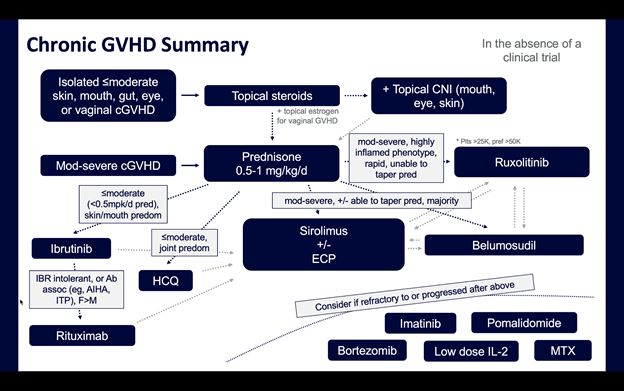
Steroids plus/minus ruxolitinib remain the mainstay of chronic GVHD management. Compared to their use in acute GVHD, steroid and ruxolitinib tapers in chronic GVHD are even slower. Among other medications for chronic GVHD, you may recognize names from other clinics across hematology: ibrutinib, ruxolitinib, rituximab, sirolimus, and more. In general, these medications are most helpful to allow for daily corticosteroids to be tapered (and hopefully stopped). Below are a few notes on these medications:
- Ibrutinib: This was the first drug ever to receive an FDA label for steroid-refractory chronic GVHD based on a phase Ib/IIa open-label study23 with historical controls.
- Ruxolitinib: Making its second appearance is this drug that just won’t quit. Ruxolitinib was approved by the FDA last year for steroid-refractory chronic GVHD based on the randomized REACH-3 trial.24
- Belumosudil: This inhibitor of the pro-fibrotic ROCK2 pathway gained FDA approval in July 2021 for steroid-refractory chronic GVHD after at least two lines of therapy (unlike ruxolitinib or ibrutinib, which can be used in the second line). Belumosudil’s claim to fame is that it is the first drug developed specifically for GVHD rather than being borrowed from another indication or pathway.
- Extracorporeal photopheresis (ECP): This isn’t actually a medication but a procedure (often done a few times per month via a central line) whereby circulating white blood cells are removed from the circulation, “treated” with 8-methoxypsoralen and ultraviolet light, and returned. How does this treat chronic GVHD? Great question with complicated answers as summarized in an article by Dr. Jennifer Schneiderman.25
Even more than with acute GVHD, multidisciplinary supportive care26 is imperative to managing chronic GVHD. For example, with ocular GVHD, ophthalmology referral is integral for punctal plugs, autologous serum tears, or fitted scleral lenses (or prosthetic replacement of the ocular surface ecosystem lenses27), which help to protect the tear film and are a game-changer for quality of life. Similarly, for vulvovaginal GVHD, topical lubricants as well as gynecological referral for vaginal dilators are warranted.
VOD/SOS
VOD/SOS is one of the first major complications to consider in the weeks following allogeneic HSCT. However, the reason for developing such complication is only partially understood. The prevailing hypothesis is that this syndrome is caused by injury to sinusoidal endothelial cells and zone 3 hepatocytes from conditioning and prior chemotherapy in the context of an alloimmune milieu, but a range of other endovascular complications may be involved such as capillary leak syndrome, transplant-associated microangiopathy, and others.
If you remember nothing else about VOD/SOS, remember that it is relatively rare but also very lethal. Estimates of its incidence vary, but one large meta-analysis reported an incidence of 13.7 percent.28 However, incidence rates are falling as the field moves towards reduced intensity and safer conditioning regimens. Recognition and early treatment of severe disease remains critical because of mortality rates exceeding 75 percent with severe disease when managed with supportive care alone.
How is VOD/SOS defined?
VOD/SOS definitions were first standardized in the 1980s, most notably with the Baltimore criteria29 and modified Seattle Criteria.30 More recently, the European Society for Blood and Marrow Transplantation (EBMT) published expanded diagnostic criteria31 that include late-onset disease as well as severity grading (Tables 4 and 5). There are some variations between criteria, but the general principles are the same.
In brief, diagnostic criteria involve some combination of elevated total bilirubin, (painful) hepatomegaly, ascites, and weight gain following allogeneic HSCT. As noted, classical VOD/SOS always occurs within 21 days of HSCT and does not require a liver biopsy or ultrasound/Doppler findings for diagnosis. In contrast, late-onset VOD/SOS can be established with liver biopsy, positive ultrasound/Doppler findings plus other signs, or classical criteria albeit a delayed onset.
Table 4. EBMT-2016 criteria for VOD following HSCT
| Classical VOD/SOS | Late-Onset VOD/SOS | |
| Diagnostic criteria |
Timing - and - Elevated bilirubin - and - 2+ of the following (hepatomegaly, weight gain, ascites) |
Meets classical criteria for VOD/SOS, minus timing - or - Biopsy-proven VOD/SOS - or - Positive ultrasound findings and 2+ of the following (elevated bilirubin, hepatomegaly, weight gain, ascites) |
| Timing | ≤ Day +21 | > Day +21 |
| Total bilirubin (mg/dL) | ≥ 2.0 | ≥ 2.0 |
| Painful hepatomegaly | Yes | Yes |
| Weight gain despite optimized diuretics | > 5% | > 5% |
| Ascites | Yes | Yes |
| Liver biopsy | Not required | See above |
| Liver ultrasound | Not required | See above |
Abbreviations: HSCT, hematopoietic stem cell transplantation; mg/dL, milligrams per deciliter; SOS, sinusoidal obstruction syndrome; VOD, veno-occlusive disease.
Table 5. EBMT-2016 severity grading for VOD/SOS after HSCT
| Mild | Moderate | Severe | Very Severe | |
| Time from Onset of First VOD/SOS Symptoms | > 7 days | ≤ 7 & > 5 days | ≤ 4 days | Any time |
| Total Bilirubin (mg/dL) | ≥ 2.0 & < 3.0 | ≥ 3.0 & < 5.0 | ≥ 5.0 & < 8.0 | ≥ 8.0 |
| Bilirubin Kinetics | Doubling w/in 48hrs | |||
| Transaminases+ | < 2× ULN | > 2× & ≤ 5× ULN | > 5× & ≤ 8× ULN | > 8× ULN |
| Weight Gain* | < 5% | ≥ 5% & < 10% | ≥ 5% & < 10% | ≥ 10% |
| Renal Function^ | < 1.2× | ≥ 1.2× & < 1.5× | ≥ 1.5× & < 2× | ≥ 2× |
+Multiple of the upper limit of normal (ULN); *After optimized diuretics; ^Relative to pre-transplant baseline.
Abbreviations: HSCT, hematopoietic stem cell transplantation; mg/dL, milligrams per deciliter; SOS, sinusoidal obstruction syndrome; ULN, upper limit of normal; VOD, veno-occlusive disease.
What are risk factors for VOD/SOS?
The most important risk factors32 for VOD/SOS to remember are:
- Prior exposure to antibody-drug conjugates (ADC) such as gemtuzumab ozogamicin (a CD33-binding ADC in acute myelogenous leukemia [AML]) or inotuzumab ozogamicin (a CD22-binding ADC in acute lymphoblastic leukemia [ALL])
- Myeloablative conditioning, most notably involving busulfan or thiotepa
- Pretransplant liver disease or elevated transaminases at baseline
Other significant risk factors for developing VOD/SOS include particular genotypes related to busulfan pharmacokinetics (e.g., with glutathione transferase genes33) or baseline hyperferritinemia. The risk of VOD may also be greater with higher degrees of donor mismatch or with second allogeneic HSCT. Sirolimus use has been associated with VOD/SOS.
How do we prevent VOD/SOS?
Part of VOD/SOS prevention involves mitigation of the risk factors above. For patients with AML or ALL where HSCT is planned, gemtuzumab and inotuzumab are often avoided respectively, or timed sufficiently in advance of transplantation. Similarly, for patients with known liver disease, reduced-intensity conditioning (and particularly the omission of busulfan) can be considered. In patients with known bridging fibrosis or cirrhosis, transplant is often avoided because of high transplant-related mortality related to VOD/SOS and GVHD of the liver.
Once a patient has undergone HSCT, a mainstay of management is ursodeoxycholic acid (ursodiol), a synthetic bile salt first identified in bears. Ursodiol seems to only have minimal to moderate efficacy in this setting,34 but its use is ubiquitous because it is generally well tolerated without significant adverse effects. Conversely, heparinization as prophylaxis has fallen out of practice given an increased risk of bleeding without a clear correlation in VOD/SOS risk reduction. A randomized study of defibrotide35 (a porcine product with fibrinolytic and anticoagulant properties) as prophylaxis in the pediatric setting showed numerical suggestions of benefit. A separate defibrotide prevention phase III study36 in adults did not demonstrate efficacy.
How do we treat VOD/SOS?
While defibrotide prophylaxis is not the standard of care, defibrotide treatment for severe and very severe disease (per the EBMT-2016 criteria) is more routine. Its FDA approval for this purpose was based on a single-arm trial37 that compared defibrotide to historical controls. Patients on defibrotide must be monitored closely for bleeding. Its use is recommended for at least 21 days or longer until symptom resolution. The cost effectiveness38 of defibrotide has been controversial39 due to the high cost for a 21-day course (~$160,000), though three separate analyses40 reported cost effectiveness, including one in the U.S. with an incremental cost-effectiveness ratio of $47,736 per quality-adjusted life-year.41
Regardless of defibrotide usage, supportive care is imperative for patients with VOD/SOS. Important steps include careful management of nutrition and renal function. Corticosteroids, our mainstay for many conditions in HSCT (including GVHD as defined below), have unfortunately not clearly demonstrated efficacy in the treatment of VOD/SOS.42 Similarly, transjugular intrahepatic portosystemic shunting procedures have not been shown to improve outcomes.
Conclusion
Allogeneic HSCT involves much more than just the bone marrow. While HSCT offers the possibility of cure for many malignant and nonmalignant hematologic conditions, these toxicities must be kept in mind in the months and years following transplantation. Below are some closing points to keep in mind:
- Inflammation is both our ally and our foe: Transplantation requires the Janusian understanding of inflammation as both a tool in the case of GVT and an obstacle in the case of GVHD. As such, steps to lower GVHD must be balanced with the risk of lowering antitumor responses.
- HSCT toxicities are, by and large, clinical diagnoses: There are many risk calculators and algorithms available to help us detect GVHD, VOD, and more. No calculator or single test — not even a biopsy — is foolproof for making the diagnosis.
- Each center handles toxicity prophylaxis and management differently: We say this so that you’re not surprised if you proceed to a rotation or fellowship at a different institution. Institutional inertia is often a big factor in why, for example, some centers use total body irradiation more routinely than others as part of conditioning. For most of the toxicities discussed above, our evidence is largely based on such single-center experiences and not large, randomized trials.
Interested in learning more about these fascinating topics? Check out the “Fundamentals of Transplantation” course offered each year by ASTCT or a dedicated one-year advanced fellowship focused on HSCT and other cellular therapies, which both of us have personally taken. Feel free to reach out to your mentors or to us if you have any questions. Thank you for reading!
Acknowledgments
We would like to thank Dr. Corey Cutler for providing thoughtful feedback to improve this article. We would also like to thank Dr. Aaron Logan for the chronic GVHD algorithm shown above.
- Satlin MJ, Weissman SJ, Carpenter PA, et al. American Society of Transplantation and Cellular Therapy Series, 1: Enterobacterales infection prevention and management after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:108-114.
- Dadwal SS, Hohl TM, Fisher CE, et al. American Society of Transplantation and Cellular Therapy Series, 2: Management and prevention of aspergillosis in hematopoietic cell transplantation recipients. Transplant Cell Ther. 2021;27:201-211.
- Hakki M, Aitken SL, Danziger-Isakov L, et al. American Society for Transplantation and Cellular Therapy Series: #3-Prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:707-719.
- Yong MK, Shigle TL, Kim YJ, et al. American Society for Transplantation and Cellular Therapy Series: #4 – Cytomegalovirus treatment and management of resistant or refractory infections after hematopoietic cell transplantation. Transplant Cell Ther. 2021;27:957-967.
- Williams KM. Noninfectious complications of hematopoietic cell transplantation. Hematology Am Soc Hematol Educ Program. 2021;2021:578-586.
- Buchbinder D, Khera N. Psychosocial and financial issues after hematopoietic cell transplantation. Hematology Am Soc Hematol Educ Program. 2021;2021:570-577.
- Bhatia S. Caring for the long-term survivor after allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2014;2014:495-503.
- Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoraic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262-1279.
- Yanik GA, Horowitz MM, Weisdorf DJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20:858-864.
- Wolff D, Lawitschka A. Chronic graft-versus-host disease. The EBMT Handbook. 2018;44:331-345.
- Kharfan-Dabaja MA, Kumar A, Ayala E, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietc cell transplantation: A report on behalf of the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2021;27:642-649.
- Bader P, Niethammer D, Willasch A, et al. How and when should we monitor chimerism after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35:107-119.
- Zijlmans JM, Visser JWM, Laterveer L, et al. The early phase of engraftment after murine blood cell transplantation is mediated by hematopoietic stem cells. Proc Natl Acad Sci U S A. 1998;95:725-729.
- Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:537-543.
- Scott AP, Tey AK, Butler J, et al. Diagnostic utility of endoscopy and biopsy in suspected acute gastrointestinal graft-versus-host disease after hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. 2018;24:1294-1298.
- Harris AC, Young R, Devine S, et al. International, multi-center standardization of acute graft-versus-host disease clinical data collection: a report from the MAGIC consortium. Biol Blood Marrow Transplant. 2016;22:4-10.
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389.e1-401.e1.
- Soiffer RJ, Kim HT, McGuirk J, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003-4011.
- Bonifazi F, Rubio MT, Bacigalupo A, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020;55:1093-1102.
- FDA approves abatacept for prophylaxis of acute graft versus host disease. FDA. 2021.
- Pidala J, Hamadani M, Dawson P, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135:97-107.
- Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382:1800-1810.
- Waller EK, Miklos D, Cutler C, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant. 2019;25:2002-2007.
- Zeiser R, Polverelli N, Ram R, et al. Ruxolitinib for glucocorticoid-refractory chronic graft-versus-host disease. N Engl J Med. 2021;385:228-238.
- Schneiderman J. Extracorporeal photopheresis: cellular therapy for the treatment of acute and chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2017;2017:639-644.
- Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167-1187.
- Theophanous C, Irvvine JA, Parker P, et al. Use of prosthetic replacement of the ocular surface ecosystem scleral lenses in patients with ocular chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21:2180-2184.
- Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157-168.
- Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778-783.
- McDonald GB, Sharma P, Matthews DE, et al. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116-122.
- Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51:906-912.
- Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transplant. 2019;25:1271-1280.
- Ansari M, Rezgui MA, Théoret Y, et al. Glutathione S-transferase gene variations influence BU pharmacokinetics and outcome of hematopoietic SCT in pediatric patients. Bone Marrow Transplant. 2013;48:939-946.
- Tay J, Tinmouth A, Fergusson D, et al. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:206-217.
- Carbacioglu S, Cesaro S, Faraci M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haematopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301-1309.
- Study comparing efficacy and safety of defibrotide vs best supportive care in the prevention of hepatic veno-occlusive disease in adult and pediatric patients. ClinicalTrials.gov. 2022;NCT02851407.
- Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656-1665.
- Belsey J, Ngonga Kemadjou E, Isaila M, et al. Cost-effectiveness of defibrotide for the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) with multi-organ dysfunction (MOD) post-hematopoietic stem cell transplantation (HSCT) in Canada. Blood. 2018;132(Supplement 1):4702.
- Carcedo Rodriguez D, Artola Urain T, Chinea Rodriguez A, et al. Cost-effectiveness analysis of defibrotide in the treatment of patients with severe veno-occlusive disease/sinusoidal obstructive syndrome with multiorgan dysfunction following hematopoietic cell transplantation in Spain. J Med Econ. 2021;24:628-636.
- Gratwohl A. Cost-effectiveness of defibrotide for treatment of severe veno-occlusive disease: it is time for evidence based economic evaluations. J Med Econ. 2021;24:727-729.
- Veenstra DL, Guzauskas GF, Villa KF, et al. The budget impact and cost-effectiveness of defibrotide for treatment of veno-occlusive disease with multi-organ dysfunction in patients post-hematopoietic stem cell transplant. J Med Econ. 2017;20:453-463.
- Mohty M, Malard F, Abecasis M, et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group. Bone Marrow Transplant. 2020;55:485-495.