Myeloid Malignancy Precursor Conditions: What Fellows Need to Know


Articles in Hematopoiesis are written for trainees by trainees, under the oversight of the ASH Trainee Council. The material published in Hematopoiesis is for informational purposes only. The opinions of the authors are their own and do not necessarily represent the official policy of the American Society of Hematology. ASH does not recommend or endorse any specific tests, physicians, products, procedures, or opinions, and disclaims any representation, warranty, or guarantee as to the same. Reliance on the information provided in this publication is solely at your risk.
 Illustrative Patient Case
Illustrative Patient Case
The patient is a 72-year-old man who presented to an outpatient clinic reporting fatigue, shortness of breath, and chest pain with exertion. Medical history was significant for benign prostatic hyperplasia but no history of abnormal blood counts or of blood transfusions. Past surgical and family history were unremarkable. He reported having one to two glasses of wine per day and had a 12 pack per year smoking history prior to quitting 20 years ago. He was actively taking aspirin, atorvastatin, and doxazosin. His vitals and physical exam were normal.
On routine labs, he had macrocytic anemia (hemoglobin, 12.9 g/dL; mean corpuscular volume, 106 µm3) with an elevated absolute reticulocyte count (58,000/µL), normal white blood cell count, and normal platelet count. Chemistries, kidney and liver function, iron studies, hemolysis labs, folate, vitamin B12, and serum protein electrophoresis were all within normal limits. The patient underwent a bone marrow biopsy, which revealed normocellular bone marrow with trilineage hematopoiesis, minor erythroid cell abnormalities, and 1 percent blast cells, with insufficient changes for a diagnosis of myelodysplastic syndrome (MDS). His cytogenetics showed a normal male karyotype. He was monitored with a complete blood count (CBC) every three months.
Nine months later, his hemoglobin began to downtrend and he developed mild neutropenia. The patient underwent a second bone marrow biopsy that revealed marked hypercellularity with granulocytic hyperplasia and 4 percent blasts. Cytogenetics showed trisomy 8, and a next-generation sequencing (NGS) panel identified mutations in ASXL1 (variant allele frequency [VAF], 43%), BCOR (VAF, 43%), U2AF1 (VAF, 23%), TET2 (VAF, 22%), and EZH2 (VAF, 91%). Morphological features were still insufficient to establish a diagnosis of MDS. Thus, he was diagnosed with clonal cytopenias of uncertain significance (CCUS).
What are Myeloid Precursor Conditions?
The development of a myeloid malignancy such as MDS or acute myeloid leukemia (AML) involves a progression through several precursor disease states (Figure 1 and Table). The earliest identifiable development in this sequence is clonal hematopoiesis of indeterminate potential (CHIP). Clonal hematopoiesis (CH) occurs when a hematopoietic stem cell acquires a somatic mutation in a leukemia-associated gene, which leads to enhanced fitness and clonal expansion relative to other stem cells. CHIP is defined by the presence of somatic mutations that are associated with hematologic malignancies in individuals without evidence of an underlying hematologic malignancy or cytopenias.1 There are several characteristic gene mutations seen in CHIP, with the most common being DNMT3A, TET2, and ASXL1 (i.e., DTA mutations).1 CHIP is a common condition that occurs in approximately 5 percent of healthy individuals aged 60 to 69 years and in nearly 10 percent of healthy individuals aged 70 to 79 years.2,3
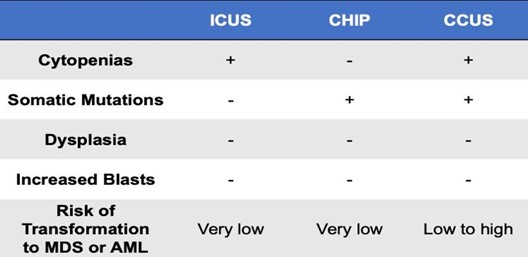
The development of cytopenias in one or more blood cell lineages is a distinguishing feature of clonal cytopenias of unknown significance (CCUS) and represents the next step in progression to a myeloid malignancy. In contrast to CHIP, CCUS is associated with clonality and ineffective hematopoiesis, leading to cytopenias in one or more blood cell lineages. Like CCUS, idiopathic cytopenia of unknown significance (ICUS) also presents with a cytopenia in one or more blood cell lineages and does not meet criteria for MDS. Although both result in cytopenias, ICUS is a nonclonal process and can be differentiated from CCUS based on the absence of somatic driver mutations. This distinction is important given the significantly poorer prognosis associated with CCUS.1
Figure 1. Stepwise Progression of Myeloid Precursor Conditions to Acute Myeloid Leukemia
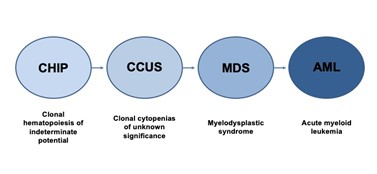
Table. Distinguishing characteristics of myeloid precursor conditions. Adapted from Osman A et al (2021).1
Risks Associated With Myeloid Precursor Conditions
Myeloid precursor conditions are associated with a variably increased risk of developing a hematologic malignancy. For individuals with CHIP, the risk of progression is quite low — on the order of 0.5 to 1.0 percent per year.2 Similarly, the overall risk of progression in individuals with ICUS is low, with a cumulative probability of clonal evolution of approximately 9 percent.4 Conversely, CCUS carries a much higher risk of transformation, with an 82 percent probability of progression at five years and 95 percent probability at 10 years.4
The risk of progression in CCUS is influenced by several clone metrics, including the number of mutations, the specific genes that are mutated, as well as the clone VAF.1 First, the more mutations that are observed, the higher the likelihood of a myeloid neoplasm developing.4 However, not all gene mutations carry the same risk. Although epigenetic modifier genes (DNMT3A, TET2, and ASXL1 or DTA mutations) are the most frequently mutated in CCUS, spliceosome genes such as U2AF1, SRSF2, and SF3B1 are associated with the highest risk of progression.1,4 Lastly, the size of the clone is important. A VAF greater than 10 percent has been associated with a 50-fold increase in the likelihood of progression.2 Patients with a VAF greater than 20 percent, mutations in spliceosome genes, and DTA mutations along with other mutations are considered to have a phenotype known as “high-risk CCUS” that carries a five-year cumulative probability of progression to a myeloid neoplasm of 95 percent.4
In addition to the risk of malignancy, myeloid precursor conditions, particularly CHIP, have been associated with an increase in all-cause mortality, largely related to cardiovascular causes including coronary artery disease and stroke.2,5,6 The mechanism conferring this increased risk has been hypothesized to involve dysregulation of macrophage gene transcription, augmented expression of inflammatory pathways, and promotion of atherogenesis.5 CHIP has also been associated with many other disease states including autoimmune disease, chronic obstructive lung disease, and pulmonary embolism.7-9 The association of CH with both hematologic and nonhematologic disorders remains an active area of investigation.
Management of Myeloid Precursor Conditions
The management and follow-up of myeloid precursor conditions is largely based on expert opinion, as prospective studies on this topic are currently lacking. There is continued debate regarding the disclosure and extended investigation of CH given the high prevalence in older populations and uncertainty as to the clinical consequence of many incidentally discovered clonal populations.10 Many institutions have established CHIP clinics to provide individualized counseling and management via a multidisciplinary health care team.1,10
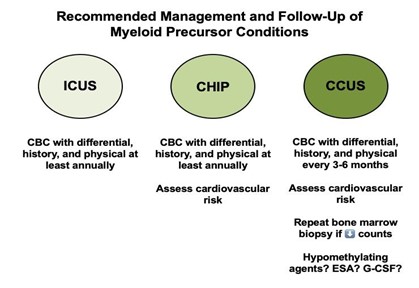
The first step in management of myeloid precursor conditions is to evaluate the patient’s risk. Patients with ICUS and CHIP should be provided reassurance, as they are unlikely to undergo malignant transformation in their lifetime. It is recommended that these patients have a CBC performed annually. Patients with CCUS should undergo more frequent monitoring with a CBC checked every three to six months, and a bone marrow biopsy should be performed if they experience a decrease in blood counts. Given the increased cardiovascular risk associated with CHIP and CCUS, providers should evaluate a patient’s risk of cardiovascular disease and manage this accordingly.1,2,5
Evidence regarding specific therapeutic interventions for high-risk CCUS is limited. One small retrospective study at a single institution showed that patients with high-risk CCUS may benefit from treatments traditionally used for myeloid disorders.11 A response rate of 78 percent was seen with the use of hypomethylating agents, and a 45 percent response rate was seen in patients treated with various growth factor agents.11 This suggests a potential benefit in treating patients with CCUS using MDS-type treatments. However, further investigation is warranted before these therapeutic strategies become a routine part of clinical practice.
Figure 2. Follow-up and Management of Patients With Myeloid Precursor Conditions
Case Resolution
The patient was diagnosed with high-risk CCUS given the presence of multiple mutations, a mutation in a spliceosome gene, and VAFs greater than 20 percent. His blood counts were monitored every three months due to his high-risk phenotype. He developed worsening cytopenias six months later, prompting a repeat bone marrow biopsy. This revealed a hypercellular marrow (80%) with increased blasts (7%), thus meeting criteria for MDS-EB-1. Cytogenetics showed a complex karyotype including trisomy 8, trisomy 14, trisomy 15, and trisomy 21. However, his NGS panel did not reveal acquisition of additional mutations. He was started on azacitidine and referred for bone marrow transplant evaluation.
- Osman AEWG. When are idiopathic and clonal cytopenias of unknown significance (ICUS or CCUS)? Hematology Am Soc Hematol Educ Program. 2021;2021:399-404.
- Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488-2498.
- Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742-752.
- Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371-3378.
- Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111-121.
- Gondek LP, DeZern AE. Assessing clonal haematopoiesis: clinical burdens and benefits of diagnosing myelodysplastic syndrome precursor states. Lancet Haematol. 2020;7:e73-e81.
- Hecker JS, Hartmann L, Rivière J, et al. CHIP and hips: clonal hematopoiesis is common in patients undergoing hip arthroplasty and is associated with autoimmune disease. Blood. 2021;138:1727-1732.
- Miller PG, Qiao D, Rojas-Quintero J, et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139:357-368.
- Soudet S, Jedraszak G, Evrard O, et al. Is hematopoietic clonality of indetermined potential a risk factor for pulmonary embolism? TH Open. 2021;5:e338-e342.
- Steensma DP, Bolton KL. What to tell your patient with clonal hematopoiesis and why: insights from 2 specialized clinics. Blood. 2020;136:1623-1631.
- Xie Z, Nanaa A, Saliba An, et al. Treatment outcome of clonal cytopenias of undetermined significance: a single-institution retrospective study. Blood Cancer J. 2021;11:43.