Workup of Possible Lymphoma: The Basics


Articles in Hematopoiesis are written for trainees by trainees, under the oversight of the ASH Trainee Council. The material published in Hematopoiesis is for informational purposes only. The opinions of the authors are their own and do not necessarily represent the official policy of the American Society of Hematology. ASH does not recommend or endorse any specific tests, physicians, products, procedures, or opinions, and disclaims any representation, warranty, or guarantee as to the same. Reliance on the information provided in this publication is solely at your risk.
 From atypical lymphocytosis, unexplained adenopathy, to full-blown B symptoms, your pathologist employs a series of steps when working up a possible lymphoproliferative disorder. Lymphocytes are supposed to proliferate, and lymph nodes enlarge for a variety of reasons; therefore, discerning a reactive versus malignant lymphoproliferation can be tricky. In this Morning Report, we present to you a cursory, practical discussion of the approach to such cases.
From atypical lymphocytosis, unexplained adenopathy, to full-blown B symptoms, your pathologist employs a series of steps when working up a possible lymphoproliferative disorder. Lymphocytes are supposed to proliferate, and lymph nodes enlarge for a variety of reasons; therefore, discerning a reactive versus malignant lymphoproliferation can be tricky. In this Morning Report, we present to you a cursory, practical discussion of the approach to such cases.
The Lymphoma Workup
The bottom line in differentiating a reactive versus a malignant lymphoid expansion is to assess for monoclonality in lymphoid cells. While there are exceptions to this rule, often a monoclonal population of lymphoid cells denotes a malignant population.
In B cells, assessing the ratio of immunoglobulin light chains kappa and lambda (the kappa:lambda ratio) and finding a monotypic process (from phenotypic studies) can be a surrogate for a monoclonal process, which is subsequently proven by polymerase chain reactin (PCR) for immunoglobulin heavy chain (IgH), kappa (Igk), and/or lambda (Igƛ). In T cells, loss of a pan T-cell antigenic marker (by phenotypic assessment) can be used as a surrogate for monoclonality, which would also be shown on PCR-based assessment. The pan-T–cell markers include CD3, CD2, CD5, and CD7 and are often retained in reactive processes. All T cells are a mixture of CD4 and CD8 cells. Absence of both, expression of both, and a marked increase in one over the other is also abnormal. It should be noted that in contrast to B-cell clones, T-cell clones can often be seen in reactive processes as well, so this information needs to be considered in context of the overall diagnostic picture.
In the remaining discussion, the objective of the studies is to determine clonality. This can be assisted by review of architectural distortion of nodal tissue, morphologic abnormalities, phenotyping with flow and immunohistochemistry (IHC), chromosomes (cytogenetics and fluorescence in situ hybridization [FISH]), and genetic studies.
Typically, the “lymphoma workup” begins with basic laboratory evaluation of peripheral blood.
Laboratory Evaluation of Peripheral Blood
The complete blood count provides not only cell counts, but also calculated values to include differentials and indices. Differentials can describe relative or absolute increases in particular cell lineages in relation to other cells or overall per cellular concentration. While many lymphomas present with absolute lymphocytosis, some may not.
Peripheral blood smear review is a vital tool. There are several morphologic clues that favor reactive versus malignant lymphocytosis. Morphologic evaluation of the spectrum of lymphoid cells may point to: Epstein-Barr virus infection (Downey-type cells with skirt-like cytoplasm), chronic lymphocytic leukemia/small lymphocytic lymphoma (lymphoma cells with “soccer ball” chromatin), hairy-cell leukemia (hair-like cytoplasmic projections), large granular lymphocyte leukemia (lymphoma cells with large granules), prolymphocytic leukemia (prolymphocytes with prominent nucleoli) – the list goes on, and these will be the subject of a subsequent Morning Report.
Phenotypic assessment of lymphoid cells can be done with flow cytometry. Flow cytometry (to be discussed in detail in another Morning Report), is a powerful technique that allows for phenotyping of unfixed cells in fluid. Briefly, the cells are individually passed in front of a laser where they are further sorted and characterized. Using fluorescently labeled antibodies, this study also provides information regarding the presence or absence of surface (or cytoplasmic) antigens such as B-, T-, and natural killer markers. The power of this evaluation is in providing data that supports a monotypic B-cell population or abnormal T-cell population. It should be noted that certain lymphomas with few malignant cells (in a mixed background) such as Hodgkin lymphomas (HLs) or T-cell/histiocyte rich large B-cell lymphomas may not be detected with flow cytometry. Additionally, in some cases of large B-cell lymphoma, the lymphoma cells burst in the process such that a monotypic population cannot be identified. Lastly, blood can also be sent for cytogenetics and FISH testing, which can be helpful in pinpointing lymphoma subclassification and even prognostication.
Tissue Investigation
Sometimes, complete studies on peripheral blood lymphocytes may provide all the diagnostic and prognostic information required for a treatment plan. However, if peripheral blood lymphocytes do not provide all the information needed, biopsy of a lymph node is the usual next step.
Nodal tissue can be obtained in several ways, ranging from small needle core biopsies to larger excisional biopsies. Often we are asked, “how much tissue will be needed?” and the answer to that question is, “It depends.” Sometimes tiny specimens can give you all the information you need, and in other cases the diagnosis is made on assessment of architecture which can be difficult to assess on small needle cores.
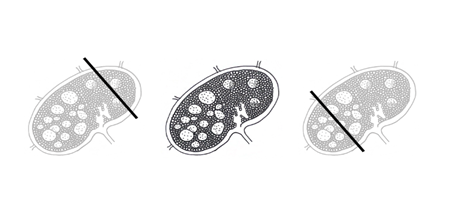
Figure 1. Needle core biopsies of lymph nodes involved in a potentially malignant process may or may not yield adequate diagnostic tissue to completely assess and diagnose a patient. In three-dimensions, a needle might “miss” the lesional proliferating cell population or may not provide adequate background architecture for a comprehensive evaluation. Left: The needle core passes through normal lymphoid tissue and will yield a result that reflects what is seen on histology. Center: This lymph node features proliferating germinal centers with normal architecture toward the top right and abnormal architecture in the bottom left. These expanded, fusing, and irregular follicles, the loss of polarity in germinal centers, and the effacement of sinusoidal areas might be consistent with follicular lymphoma, for example. Right: This needle core passes through the lymphoproliferative area and may provide more data into what might be causing the clinical lymphadenopathy.
The reason there is no “one-size-fits-all” option is because different lymphomas rely on different things to be diagnosed properly. Follicular lymphoma is diagnosed using morphology (counting centroblasts for grade), architecture (assessing nodular vs. diffuse), phenotyping by flow cytometry and IHC (germinal center origin with BCL-2 overexpression and presence of monotypic B cells), and cytogenetics (presence of t[14;18]). A needle core could give rise to the diagnosis but would not be able to rule out adjacent diffuse large B-cell lymphoma (DLBCL). Additionally, if there were less than 10 follicles, grading would be difficult. In contrast, studying classic HL by cytogenetics or flow cytometry is less useful since there are typically no abnormal findings, and morphology with appropriate IHC stains can yield the diagnosis.
A Primer on Microscopic Lymph Node Structure
Understanding the architecture of lymph nodes is crucial to creating a working lymphoma differential. Recognizing the patterns in normal, reactive, and lymphoproliferative diseases are equally important to translate histology into clinical data. When reviewing slides with your Pathologist, try and recognize the following compartments: the capsule, the cortex, the paracortex, and the medulla.
Your Pathologist is evaluating the same compartments and looking for abnormalities. Lymphoid material flows into the node from afferent lymphatic vessels, through the cortical, paracortical, and medullary sinus regions, ultimately to efferent vessels.
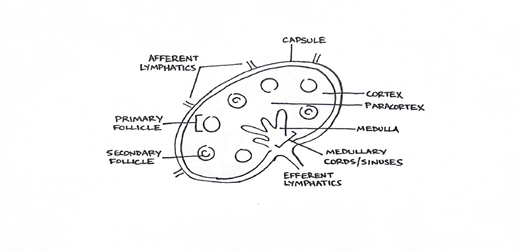
Figure 2. The location of cells within lymphoid architecture is critical to understanding the possible etiologies that might explain a patient's particular diagnosis or lymphoproliferative process.
Within the cortex are follicles comprised of mostly B cells, surrounded, and enmeshed with T cells into the paracortical region which contains more T cells along with histiocytic dendritic cells (antigen-presenting cells). Endothelial venules are also in this region. Follicles in the cortex can be primary (non-reactive) or secondary (active), whereby secondary follicles feature the growth and expansion of germinal centers of proliferation and development. Germinal centers are surrounded by marginal and mantle zones with their own B-cell/T-cell lymphoid architecture and contain active centers with various lymphoid cells. Toward the medullary sinuses and cords, the pathway of activity favors memory B cells, plasma cells, and reactive macrophages.
Knowing which cells are in which areas helps to understand various lymphoproliferative entities. For example, mantle cell lymphoma features expansions in the mantle zone with a particular lymphoid phenotype. Other lymphomas, like classic HL, DLBCL, plasma cell dyscrasias, and various T-cell lymphomas all have distinct morphologic, phenotypic, and architectural patterns.
Tissue Processing
Tissue evaluation is still one of the most critical sources of data in the workup of lymphomas. For a complete discussion, it is important to consider the processes involved in tissue processing. Since flow cytometry and cytogenetics are studies that cannot be performed on formalin-fixed tissue, the guideline from most institutions is to mark tissue for “lymphoma workup” and send tissue “fresh” in RPMI media — not in formalin. Here, the term “fresh” contrasts it to “fixed.”
Once in the pathology gross-lab, a pathology resident physician and/or a pathologist’s assistant will measure, assess, dissect, and process the nodal lymphoid tissue. They will then send it for paraffin embedding and hematoxylin and eosin (H&E) slides for histology, and touch-prep slides stained by a quick-diff method. A portion of the tissue will also be sent for flow cytometry and cytogenetics. Touch imprints of the tissue may also be made.
Touch preps are exactly what they sound like: tissue “touched” directly to a glass slide, air dried, and quickly stained for cytologic and morphologic assessments. Much like in bone marrow evaluations, lymph nodes can be touch prepped much like the core biopsies obtained in marrow collection. These nodal slides are similarly stained with a Romanowsky-type stain which allows for a detailed look into the nuclear and cytoplasmic features of potentially malignant cells.
H&E slides are critical for a myriad of diagnostic evaluations in pathology. In these sections, the pathologist can evaluate for architectural distortion, cytologic atypia, and effacements in normal histologic architecture that might allude to a particular pathologic process. These slides take time to process, from dissection to tissue fixation, embedding in paraffin wax blocks, cutting, and staining slides, and later applying additional IHC stains if they are needed; therefore, ensuring that there is adequate tissue is most helpful. These processing steps require the use of formalin to fix the tissue and must be saved for the last step as formalin would destroy viable cells/tissue required for the following ancillary studies.
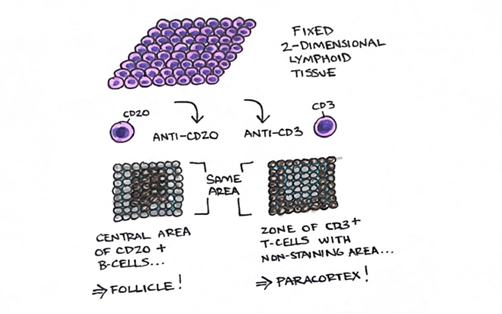
Figure 3. Fixed and processed tissue is embedded in paraffin wax and cut for slide and stain preparation; in a two-dimensional space, these slides represent “cross-sections” of tissue in vivo. Applied IHC stains with specific antibodies to cell surface markers may demonstrate architectural patters which reflect certain cell subsets. For example, the left section stained with CD20 demonstrates a central area of CD20+ cells (i.e., B cells) while the right section stained with CD3 demonstrates a zone of CD3+ cells (i.e., T cells) with a central, non-staining area. Both sections are from the same area of tissue as a histotechnologist cuts further into the paraffin wax block, micrometers at a time. The pattern illustrated here most likely demonstrates a follicle and/or germinal center with a surrounding parafollicular zone.
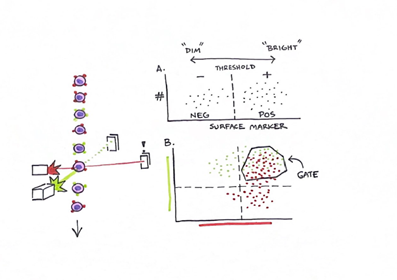
Figure 4. Flow cytometry analyzes cells as a single monolayer passing through laser instrumentation in sheath fluid. Activation of certain cell surface markers can elicit reactions which are recognized as events over time. The intensity of these events can be described as dim or bright and reflects both the amount of marker on cells' surfaces as well as the number of cells activating the event. “A” demonstrates the concept behind dim and bright while “B” represents the ability to compare multiple markers at once, often using colors, to see which markers may be “co-expressed” on cells and provide in-depth data on phenotypic profiles. Using instrumentation and software, we are also able to “gate” certain subpopulations of cells for further analysis.
Genetic/cytogenetic testing in lymphoma can be important and highly sensitive. Cytogenetics tests evaluate for abnormalities in lymphocyte chromosomes. These tests can include karyotyping or FISH for looking at chromosomes. PCR-based testing that targets DNA shows changes in even a small sample of lymphoma cells and can be helpful in determining clonality and specific abnormalities as well.
Summary
The considerations for a lymphoma workup involve a multitude of histopathologic, phenotypic, and ancillary laboratory data. The importance of all the diagnostic tests, in tandem, yield the most accurate diagnostic assessment. Understanding the processes involved in the triaging of lymphoid tissue specimens provides context in the use of hematopathologists' consultations and diagnoses. The overlap between these different testing modalities and their results contribute to our investigation of possible malignancy along with clinical correlation for your patient's lymphoma workup.