Demystifying the Bone Marrow Biopsy: A Hematopathology Primer


Articles in Hematopoiesis are written for trainees by trainees, under the oversight of the ASH Trainee Council. The material published in Hematopoiesis is for informational purposes only. The opinions of the authors are their own and do not necessarily represent the official policy of the American Society of Hematology. ASH does not recommend or endorse any specific tests, physicians, products, procedures, or opinions, and disclaims any representation, warranty, or guarantee as to the same. Reliance on the information provided in this publication is solely at your risk.

Your patient’s cytopenias remain unexplained.
Maybe a routine peripheral smear caught some circulating blasts. A serum protein electrophoresis might have even shown a monotypic expansion. Whatever the cause, you have reason to request a hematopathology workup and investigative studies. You order a bone marrow analysis for your patient.
What happens after you place the orders, though? How do the different parts of a “bone marrow workup” relate to more in-depth analyses of morphology, markers, lineages, and overall diagnostic information? Why do some investigations yield more, or less, information than others? What is the hematopathologist looking for when assembling all the parts to report back in consultation with you?
The following breakdown shines some light inside the “black box” of hematologic diagnostics and may provide insight into what the hematopathology report tells you. Herein lies everything you were afraid to ask.
The Basics
After patient preparation, sedation, and the procedure itself, a bone marrow investigation provides four specimen types for pathologist review (Figure 1): the bone marrow core biopsy, the bone marrow touch imprint, the bone marrow aspirate smear, and the bone marrow clot particle.
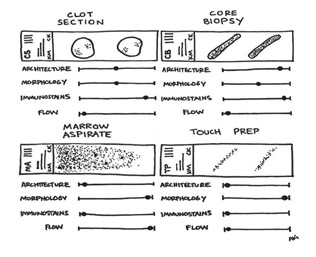
Figure 1. The four components of a routine bone marrow analysis.
The clot sections, core biopsy, marrow aspirate, and touch preps all contribute to the overall assessment of patients’ collected marrow. By using redundancies across components, your consultant hematopathologists may offer insights into the architecture, morphology, immunostaining, and flow cytometry profiles of any identified hematologic entity. Some components are more useful for particular studies.
These specimens are differentially used to study morphology, assess lineage, perform cell counts and differentials, triage and send for appropriate immunohistochemical stains, perform flow cytometry, and send ancillary cytogenetic and molecular genetic studies. Each of these four specimens have their strengths and limitations; therefore, they should be assessed separately. While their individual turnaround times vary, these specimens are usually reported together to make sure all aspects are accounted for, which can take approximately three days on average.
Unlike complete blood counts (CBCs), which produce fast results, a bone marrow analysis requires a more in-depth analysis and, as a more invasive procedure, necessitates built-in redundancies to ensure the highest-quality results. While there are advantages and disadvantages to each component regarding turnaround time, comprehensiveness, and diagnostic utility (Table), their synergism provides ample information for your consultant hematopathologists.
Table: Comparative advantages and drawbacks of the marrow aspirate versus the core biopsy.
| Marow Aspirate | Core Biopsy | |
| Advantages |
|
|
| Drawbacks |
|
|
The separation of these four components allows for multiple sources of data collection and offers insurance against otherwise compromised specimens. Hematopathologists can assess morphology, histologic architecture, and immunologic and phenotype profiles (Figure 2) across all four components to create a comprehensive report for your patient.
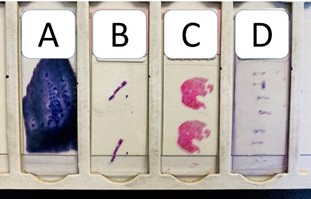
Figure 2. Picture of four bone marrow specimens in a slide tray. A complete bone marrow biopsy examination usually involves the review of these four specimens noted here in a slide tray: A) marrow aspirate smear, B) marrow core biopsy, C) clot section, and D) touch imprint preparation.
Marrow Aspirate
The bone marrow aspirate is arguably the most straightforward aspect of the bone marrow workup. A syringe with applied negative pressure gently removes approximately 5 mL of deep red, semi-liquid marrow content. This is used to immediately make slide preparations on one to 10 pre-prepared glass slides which will be stained, usually within the Giemsa family of stains, to assess cellular morphology (how the cells look), perform a lineage assessment (what cell line they belong to, both by morphology and phenotyping), and provide a complete differential count (500 cells are counted). Since this is a “liquid” sample, it does not need to undergo decalcification, can be smeared onto a slide and stained relatively quickly, used for flow cytometry (which needs unfixed, liquid cells), and sent fresh for molecular analysis. Additionally, since the cells are smeared, they are technically three-dimensional, and morphology can be assessed.
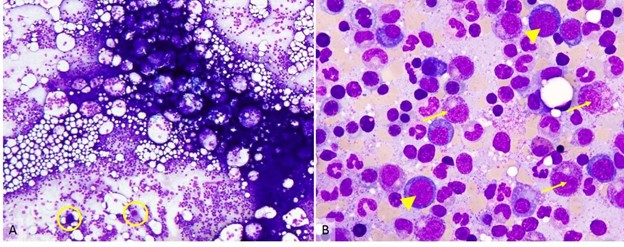
Figure 3. The bone marrow aspirate smear. Wright-Giemsa staining of the marrow aspirate smear. A) 20× view of the bone marrow aspirate reveals a deeply basophilic smear of cells. Megakaryocytes (yellow circles) can be seen at low power. B) 1,000× view. Note granulocytic precursors (arrows) and erythroid cells (arrow heads). View an interactive bone marrow aspirate online.
Determination of cellular phenotype (what the cell expresses on its surface, or in its cytoplasm) on an aspirate is carried out by flow cytometry. Aspirate slides are also used for cytochemical and iron stains. Extra smeared slides are kept unstained for possible subsequent ancillary staining (e.g., MPO, PAS, esterases).
Quick tip: A cellular aspirate smear is crucial to an adequate differential count and assessment of morphologic dysplasia. Hypocellular or paucispicular smears preclude these assessments, which are not easily (or accurately) performed on the core biopsy (Table).
Bone Core Biopsy
After the aspirate, the most expected informative component of a bone marrow workup is likely the core needle biopsy. The double needle from the same aspirate procedure is used to cut a core biopsy of optimal length (“longer is better”; i.e., more data). Once obtained, the core biopsy is used to make “touch preps” (discussed below) and then is transferred into a container with appropriate fixative (usually formalin) and sent to the laboratory for processing. The specimen is fixed in paraffin and cut for slide preparation. Due to the decalcification and need for fixation, bone core biopsy slides are usually not available to review until the following day.
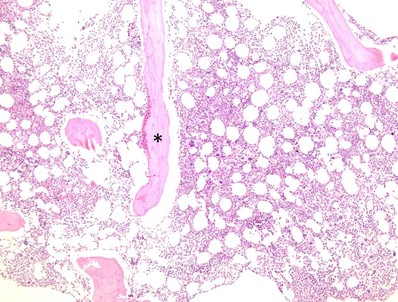
Figure 4. Bone core biopsy. Hematoxylin-eosin–stained section of the bone core biopsy (100×). Note the trabecular bone (*) with trilineage hematopoiesis including megakaryocytes, granulocytic precursors, and erythroid islands presented in 2D following formalin fixation and paraffin processing. View an interactive bone marrow core biopsy online.
The core biopsy is useful for assessing overall marrow cellularity, trilineage hematopoiesis, and marrow architecture. The prepared core biopsy slides can be used for immunohistochemical (IHC) investigations (phenotyping the cells using IHC stains), and an initial standard hematoxylin and eosin stain is done to assess baseline histology. After these initial assessments, immunostains often aim to assess architecture, fibrosis, lymphoid aggregates, myeloid lineage maturity, and other related potential pathologies. Since it is a two-dimensional specimen and reveals cells in cut section, it is not ideal for assessment of dysplasia (a marrow aspirate is preferred), but it is extremely useful in identifying possible reasons for a “dry tap” (a term for when liquid marrow cannot be aspirated during the bone marrow procedure), since the architecture can show fibrosis, sheets of cohesive plasma cells, or metastatic tumor which could result in a dry tap.
Quick tip: If the bone marrow is involved by metastatic carcinoma or clusters of cohesive plasma cells, these abnormal cells may not be amenable to aspiration and may cause a dry tap; however, a bone core biopsy will identify them. As such, if a dry tap is encountered during a bone marrow procedure, it may be beneficial to obtain two bone core biopsies to send to the laboratory.
Clot Section
Since the marrow is abundantly deep red and more viscous than blood, the red cell and platelet components will eventually form clots if no anticoagulant is present. Before the specimen is transferred to a container with anticoagulant, some of the already clotted specimen may be submitted for permanent histology in formalin. These formalin specimens are embedded in paraffin blocks and sectioned by histotechnologists to provide a two-dimensional cross-section of the clotted tissue. Unlike the core biopsy, decalcification is not required for the clot section.
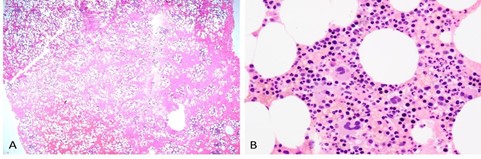
Figure 5. Low and high power of clot particle. Hematoxylin-eosin–stained sections of the clot particle. A) 20× view of the clot. Note extensive red blood cells in the background. B) 600× view of trilineage hematopoiesis. The morphology is similar to that seen on the core biopsy. View an interactive bone marrow clot specimen online.
Within these sections, there are often small areas of hematopoietic material preserved from their original marrow environment. These can be highly useful when a core biopsy is suboptimal, demonstrates marked myelofibrosis, is a dry tap, or otherwise fails to provide adequate visual data for morphology, architecture, cellularity, and hematopoietic lineage assessments. Furthermore, the clot section, like the core biopsy, can be used for immunohistochemical stains. As a two-dimensional section of “islands” of preserved marrow content, the same stains applied to the core biopsy can be applied to the clot sections.
Quick tip: Flow cytometry cannot be performed on the clot section after the clot has set and after fixation in formalin. Without individual cells to analyze through flow cytometric methods, the clot section is limited to only tissue-type immunostaining. That said, this specimen (if involved by a disease process) can be sent for genetic testing such as polymerase chain reaction and does not present the issue of being postdecalcification (which may hinder some genetic tests).
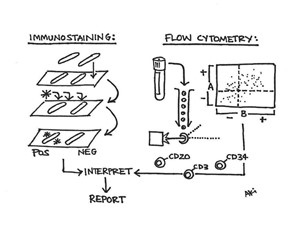
Figure 6. Phenotyping hematopoietic cells. The phenotypic composition of the various marrow components is key to understanding their utility for further investigative diagnostic studies. Two-dimensional fixed tissue specimens from the biopsy and clot are easily stained with immunohistochemical methods while three-dimensional, liquid cellular content can be assessed with flow cytometry. In part, each component is analyzed and interpreted in correlation together for a final report.
Touch Preparations
Immediately after the core biopsy is obtained, the procured tissue is "touched" several times onto glass slides. The morphologic findings are very similar to that of the aspirate smear, with the caveat that it only represents the cells that slough off.
Because of the densely cellular composition of bone marrow, the imprints impart many cells directly on the slides. Touch preps can be imprinted, rolled, or crushed between glass to provide similar information. They are then stained and processed much like the original core biopsy. Without the bone marrow matrix, these slides contain only cells directly from the marrow and can be stained and assessed both for lineage and cytologic morphology, with high correlations to what may be seen on aspirate and biopsy. Like the marrow aspirate smear, touch imprint preparations provide a quick turnaround time (i.e., do not need decalcification) and great morphologic detail (if the aspirate smears are paucispicular or hemodiluted).
Summary
In short, bone marrow analyses yield dynamic results, informing clinical diagnostics and treatment plans. As a medical procedure, bone marrow collection may sometimes have limitations in obtaining adequate specimens. As such, the redundancies in place discussed here and the compound output of the four major components have synergistic effects on diagnostic evaluation. Understanding the capabilities and potential within each component may explain both the process and usefulness of obtaining optimal specimens and elucidate exactly how tissue is evaluated.
Quick Q&As from the hematopathologists:
Q: Can your pathologist give you a preliminary assessment of the aspirate smear or flow cytometry analysis on the same day as a procedure if it was performed early in the morning?
A: Probably, yes.
Q: Can your pathologist tell you what the core biopsy shows on the same day as the procedure?
A: No
Q: Can flow cytometry be performed on the core biopsy?
A: Ideally, no. If no aspirate is collected, then an extra core biopsy specimen can be agitated to release cells for flow cytometry; however, this is not ideal.
Q: Can flow cytometry be used for assessment of morphologic dysplasia?
A: No
Q: Can the core biopsy determine the blast count?
A: Ideally, blasts should be calculated on the aspirate smear differential count; however, in cases where blasts express CD34, then a CD34 count on the core biopsy might be possible.