Case Study: 32-year old man with neurologic changes and cytopenias
A 32 year-old previously healthy male presented to the emergency department with progressive visual changes and dysarthria of several days duration. A CT scan of the brain was significant for multiple infarcts of varying chronicity involving the occipital cortex and cerebellum.
Initial laboratory investigations:
| Hemoglobin | 7.8 g/dL | (13-18 g/dL) |
| Platelet count | 20x109/L | (150-400x109/L) |
| Leukocyte count | 1.8x109/L | (4-11x109/L) |
| Neutrophil count | 0.6x109/L | (2.0-7.5x109/L) |
| Blast cell count | 0.8x109/L | |
| INR | 1.4 | (0.8-1.2) |
| PT | 16.7 | (12.1-14.4) |
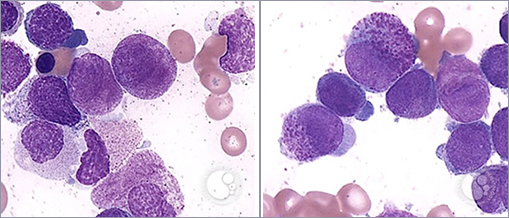
Bone marrow aspirate shows 18% promyelocytes:
| Figure 1 | Figure 2 |
 |
|
Which of the following is the most likely to be found on further testing?
- The leukemic cells typically have high expression of CD33.
- The disease is associated with t(15;17).
- The typical form of the disease is characterized by a very high leukocyte count with rapid doubling time.
- The disease is associated with t(8;21).
Answer
- The disease is associated with t(15;17).
Explanation
This patient’s clinical findings and bone marrow aspirate are consistent with a diagnosis of acute promyelocytic leukemia (APL), or acute myeloid leukemia (AML) with t(15:17)(q22;q12), in which promyelocytes predominate. A diagnosis of APL does not require the blast count to be >20% as the presence of t(15;17) confirms the diagnosis. APL comprises 5-8% of AML. In APL, the retinoic acid receptor alpha (RARA) gene on 17q12 fuses with a nuclear regulatory factor gene (promyelocytic leukemia or PML gene) on 15q22 producing a fusion gene product PML-RARA. The bone marrow aspirate images above demonstrate characteristic morphological features of hypergranular (typical) APL including large immature blast cells with high nuclear-to-cytoplasmic ratio, prominent nucleoli, reniform (folded) nuclei and abundant primary azurophilic cytoplasmic granules consistent with promyelocytes. Abnormal promyelocytes may also contain numerous Auer rods (faggot cells, not shown). The microgranular (hypogranular) type of APL is characterized by a paucity or absence of granules and predominantly bilobed nucleus. This patient has clinical and laboratory findings concerning for disseminated intravascular coagulation (DIC), which can occur with any subtype of AML, but is a frequent complication of APL.
Microgranular APL, unlike typical APL, (as shown above), is associated with a high leukocyte count and rapid doubling time. Hypergranular (typical) APL with t(15:17) is characterized by high expression of CD33, heterogeneous expression of CD13 and low expression or absence of HLA-DR, CD34, CD11a, CD11b and CD18. APL is not associated with t(8;21).
Induction therapy for APL includes all-trans retinoic acid (ATRA) which promotes differentiation of malignant promyelocytes. ATRA is usually combined with anthracycline-based chemotherapy. Recent evidence suggests that for patients with low/intermediate risk APL induction with ATRA and arsenic trioxide (ATO) is at least equivalent to standard regimens combining ATRA and chenmotherapy with reduced myelosuppression and cardiotoxicity1. Differentiation syndrome (retinoic acid syndrome or cytokine storm) is a complication seen in approximately 25% of patients with APL treated with ATRA or ATO typically occurring 10-12 days after treatment initiation (range 2-46 days). It is more common among patients with high leukocyte counts at diagnosis (WBC 10K). Clinical features include fever, respiratory distress, hypoxemia, pulmonary infiltrates, pleural or pericardial effusions, hypotension, acute renal failure, peripheral edema and hepatic dysfunction. Early recognition and prompt initiation of steroids (dexamethasone 10 mg every 12 hrs) is critical and appears to reduce mortality associated with differentiation syndrome.
Case study submitted by Deborah Siegal, MD, MSc, FRCPC, Clinical Scholar, Division of Hematology and Thromboembolism, McMaster University, Hamilton, ON.
Reference
- Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013 369(2):111