ASH Guidelines on Use of Anticoagulation in Patients with COVID-19
ASH has issued recommendations for the use of anticoagulation in critically and acutely ill patients, and for patients being discharged from the hospital. Access the full guidelines on the Blood Advances website:
Anticoagulation in COVID-19 Algorithm
Guideline IMPLEMENTATION Tools and Resources
ASH guidelines are reviewed annually by expert work groups convened by ASH. Resources derived from guidelines that require updating are removed from the ASH website.
Visual Abstracts


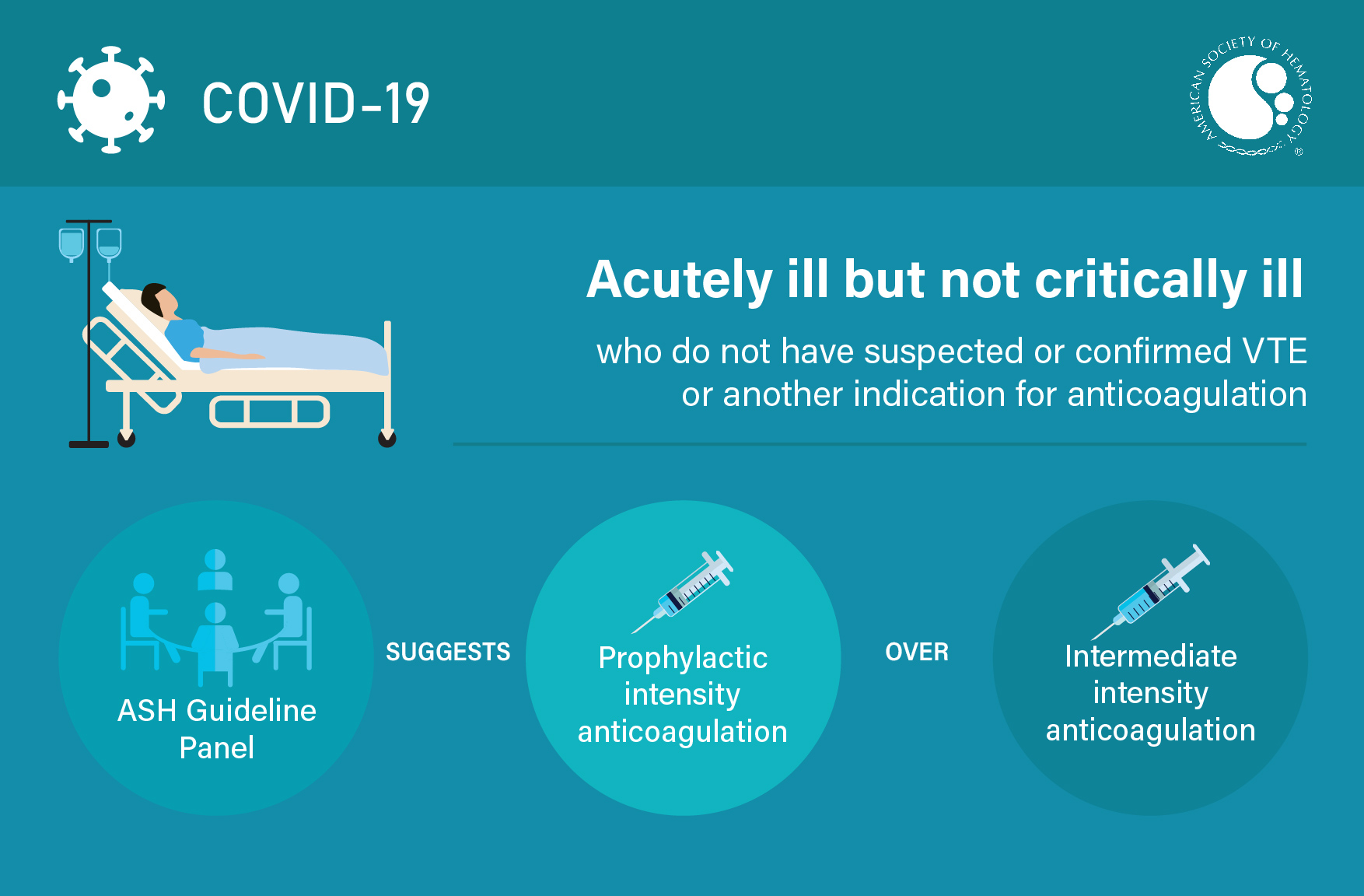
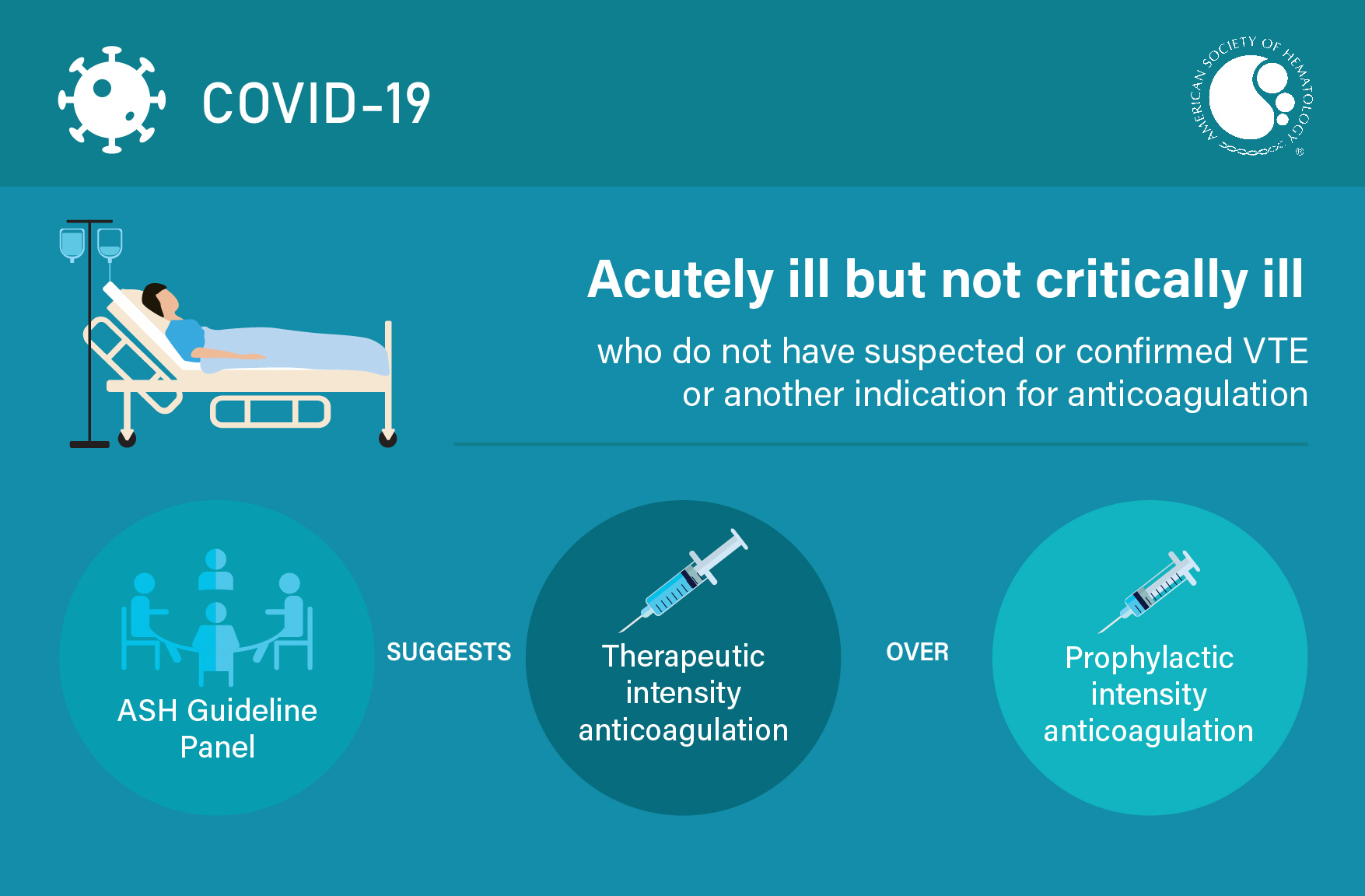
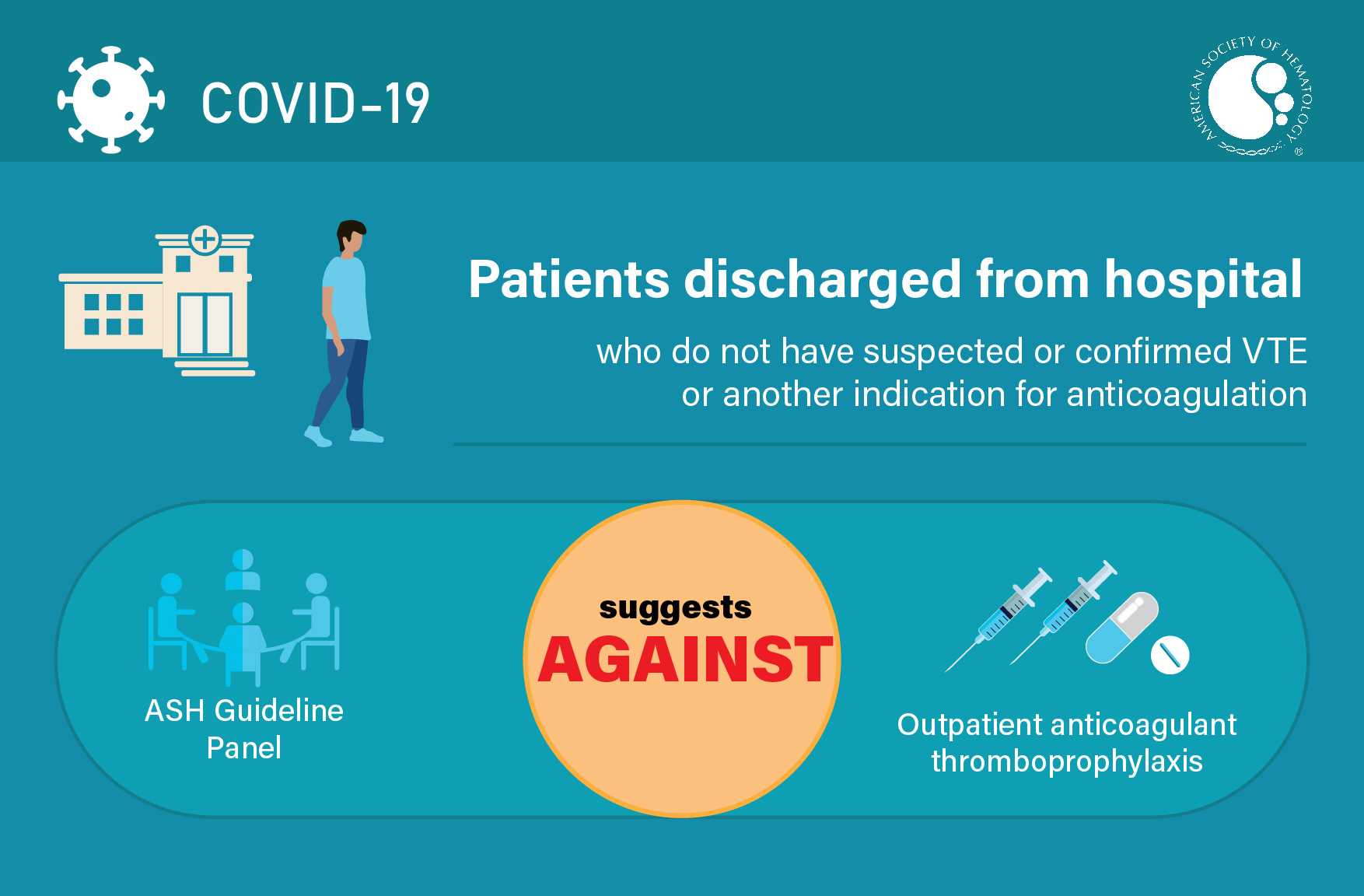
TEACHING SLIDES
Teaching slides for the Use of Anticoagulation in Patients with COVID-19
ASH Clinical Practice Guidelines App
Easy mobile- and web-access to every recommendation from all guidelines published by ASH.
Access via web Download for iOS Download for Android
Pocket Guides
Brief, evidence-based pocket guides to help physicians provide quality care to patients.
