General Principles of COVID-19 Vaccines for Immunocompromised Patients
While we regularly review and update the information provided here, this FAQ reflects only the best information available at a given point in time. The COVID-19 pandemic is a rapidly evolving global health crisis and new information may have become available since the revision date for each published FAQ.
(Version 6.1; last updated April 14, 2022)
Input from Jeff Auletta, MD; Michael Boeckh, MD; Cindy Dunbar, MD., PhD, Maheen Z. Abidi, MD; Roy F. Chemaly, MD; Fareed Khawaja, MD; Mini Kamboj, MD; Genovefa Papanicolaou, MD; Josh Hill, MD; Julie Kanter, MD; Alpana Waghmare, MD; Adrian Wiestner, MD; John R. Wingard, MD, Jane Winter, MD; Laura C. Michaelis, MD; Sanjeet Dadwal; MD; Zeinab El Boghdadly, MD; and Tobias Hohl, MD, PhD.
Please see specific FAQ for guidance on vaccination in patients who have received HCT or CAR T cells. Please see the FAQ dedicated to adverse effects related to adenoviral vector vaccines for the most up-to-date recommendations related to vaccines and clotting risk.
In the United States, two novel messenger RNA (mRNA) vaccines and one novel adenovirus vector- based vaccine have been approved through the U.S. Food and Drug Administration’s (FDA’s) Emergency Use Authorization (EUA; Figure). The BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 (Moderna) COVID-19 vaccines have both been shown in large phase III clinical trials to be more than 90 percent effective at preventing lab-confirmed COVID-19 illness and severe infections.1,2 The single-dose recombinant, replication-incompetent adenovirus serotype 26 vector-based vaccine (Ad26.COV2.S; Johnson& Johnson/Janssen) reduced the incidence of symptomatic COVID-19 with a reported overall efficacy of 66.1 percent (72% in the United States) based on data from the phase III clinical trial.3 The overall lower efficacy was thought to be due to the then newly emerging SARS-CoV-2 variant arising from South Africa (20H/501Y.V2 variant [B.1.351]), which was the predominant strain circulating in South Africa at the time of the clinical trial and accounted for 95 percent of the sequenced isolates.3 Data on efficacy of vaccines in additional variants are accumulating.
Table. COVID-19 Vaccination Schedule for People Who Are Moderately or Severely Immunocompromised*
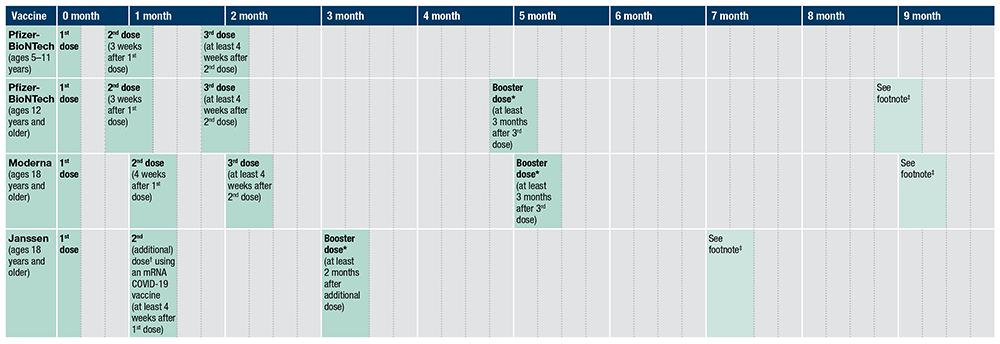
Note: Timeline is approximate. Intervals of three months or fewer are converted into weeks per the formula “1 month = 4 weeks.” Intervals of 4 months or more are converted into calendar months.
*Adapted from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. Accessed April 14, 2022. An mRNA COVID-19 vaccine is preferred over the Janssen COVID-19 Vaccine for booster vaccination of people ages 18 years and older. For people ages 12 to 17 years, only Pfizer-BioNTech can be used. A booster dose is not authorized for people ages five to 11 years.
†Only Pfizer-BioNTech or Moderna COVID-19 Vaccine should be used. See Appendix D for more information on vaccinating people who are moderately or severely immunocompromised and who received Janssen COVID-19 Vaccine for the primary series.
‡People ages 12 years and older may choose to receive a second booster dose using an mRNA COVID-19 vaccine if it has been at least four months after the first booster dose. For people ages 12 to 17 years, only Pfizer-BioNTech can be used.
What are mechanisms of action for the currently available SARS-CoV-2 vaccines?
The currently available SARS-CoV-2 vaccines use novel mechanisms of action to elicit an immune response in patients. Conventional methods included administration of attenuated inactivated (killed) virus or recombinant viral protein vaccines to develop immunity. However, the novel approaches include replication-deficient, adenovirus vector-based vaccines that contains the SARS-CoV-2 spike protein4,5 and mRNA-based vaccines that encode for a SARS-CoV-2 spike protein.1,2 Based on phase I/II and III studies, SARS-CoV-2 vaccines using the mRNA vaccine platform or the adenovirus vector-based platform elicited both humoral and cellular immune responses.
How are the SARS-CoV-2 vaccines administered and what challenges exist for vaccination?
Available mRNA COVID-19 vaccines require three separate inoculations separated by three to four weeks. Current challenges for an approved SARS-CoV-2 vaccine include manufacture to scale, distribution, storage conditions, reconstitution, and administration, particularly for the lipid nanoparticle mRNA-based vaccines, which require low temperatures for adequate vaccine preservation. Most importantly, the public must be willing to receive the vaccine.
What SARS-CoV-2 vaccines are approved for use in immunocompromised patients?
Despite several vaccine candidates being in phase II/III clinical trials, data on vaccine efficacy and safety in immunocompromised patients remain scarce; there are ongoing trials at this time. Efficacy of SARS-CoV-2 vaccines remain to be defined in immunocompromised patient populations, though emerging literature in patients with cancer and in stem cell transplant and solid organ transplant recipients suggests that mRNA vaccines are safe. There are no data that preferentially support one vaccine over another in this or any population.
Why might some hematology patients not respond to vaccines?
In order to generate optimal protective immunity following vaccination, intact host immunity is needed, particularly with respect to antigen presentation, B- and T-cell activation, and plasma B-cell antibody generation. Therefore, hosts lacking functional adaptive immune cells may be unable to generate a fully protective immune response to a SARS-CoV-2 vaccine approved for use in the general population.
The following immunocompromised patient populations could have attenuated or absent response to SARS-CoV-2 vaccines:
- Primary and secondary immunodeficiencies involving adaptive immunity
- Splenectomy or functional asplenia
- B-cell directed therapies (e.g., blocking monoclonal antibodies against CD20 or CD22, bispecific agents like blinatumomab, CD19- or CD22-directed chimeric antigen receptor T cell [CAR-T] therapies, Bruton tyrosine kinase [BTK] inhibitors)
- T-cell-directed therapies (e.g., calcineurin inhibitors, antithymocyte globulin, alemtuzumab)
- Many chemotherapy regimens
- High-dose corticosteroids (>2 mg/kg/d daily prednisone for greater than two weeks, or equivalent)
- Hematopoietic cell transplantation (HCT), especially within the first three to six months after autologous HCT and often longer after allogeneic HCT
- Underlying aberrant immunity (e.g., graft-vs.-host disease, graft rejection, absent or incomplete immune reconstitution, neutropenia ANC <500/μL, lymphopenia ALC <200/μL)
What are the current recommendations for providing a third dose of mRNA vaccine or a booster?
Due to evidence that immunocompromised patients may not mount a sufficient immune response to a two-dose primary series of either mRNA vaccines or a single-dose adenovirus vector–based vaccine, additional doses have been recommended by the Centers for Disease Control and Prevention (CDC) as of February 2022.
A third (additional dose) and fourth (booster) dose of either of the mRNA vaccines have been approved by both the FDA and CDC for immunocompromised patients. After the receipt of the original series of either of the two mRNA vaccines, a third dose is recommended 28 days or more after the second dose. Furthermore, the time between the third dose and the booster dose (the fourth dose) was reduced from five to three months for recipients of the mRNA vaccines. Patients who received the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) are eligible to receive a second dose of the mRNA vaccine 28 days or more after the first dose, followed by a booster dose two months after the second dose. The booster can be either the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) or the mRNA vaccine. This is elaborated in the Figure.
Are pediatric patients eligible to receive the third vaccine dose?
FDA approval or EUA applies to patients five years or older for Pfizer vaccine and 18 or older for Moderna.
There is, potentially, a low risk for myopericarditis in adolescent patients, usually occurring after receiving second dose BNT162b2 mRNA vaccine. Whether additive risk for cardiotoxicity occurs in patients receiving cardiotoxic chemotherapeutics like anthracyclines or after receiving additional mRNA vaccine dosing is unknown at this time.
Which vaccines can be used for a third dose or fourth dose (booster)?
Immunocompromised patients who receive either of the mRNA vaccines require three doses of the vaccine to complete their primary series. A booster or fourth dose can be given three months from the last dose. Mixing mRNA vaccines or heterologous vaccination is not recommended at this time, but if vaccine centers are unable to provide the vaccine of preference interchanging between mRNA vaccines is safe. mRNA are the preferred vaccination method.
Regarding patients who were vaccinated with the single-dose Ad26.COV2.S vaccine (Johnson & Johnson/Janssen), the CDC recommends that immunocompromised patients get a second dose of either mRNA vaccine to complete their primary series two months after their first dose and a third dose (booster) with the mRNA or Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) two months after the second dose.
Should I use serological testing to decide if my patient should receive the third vaccine dose?
Serological testing before the third dose is not recommended at this time. The currently available assays are non-standardized, and the clinical thresholds of protection are not known.
My patient’s serological test is positive for anti-spike antibodies. Should they receive the third dose?
The third dose should be administered regardless of serological results or antibody levels if the patient meets eligibility criteria.
Should patients with breakthrough infection receive the third dose?
Although the safety of additional doses in patients with breakthrough infection is not known, a third additional dose or a booster dose (fourth dose) should be considered as per the CDC recommendations after patients recover from their infection. Specifically, patients who were within six months of HCT or CD-20 therapy at the time of the primary vaccine series should receive the third dose after a breakthrough infection.
Are patients who received monoclonal antibody (mAb) for post-exposure prophylaxis eligible to receive the third dose of the vaccine?
Yes, no deferral period is recommended after the mAb treatment.
Should patients who are diagnosed with COVID-19 or exposed to someone with COVID-19 receive mAb if they have already received three doses of the vaccine?
Yes, mAb that works against the circulating variant, when or if available, should be administered regardless of previous vaccination or number of doses received.
What is known about the safety and efficacy of protein-based or killed (inactivated virus) vaccines in immunocompromised patients?
Vaccine safety encompasses acute and long-term adverse effects associated with a vaccine. Based on experience with other recombinant protein-based and inactivated (killed) virus-based vaccines, no major adverse effects or unique adverse effects have been reported in immunocompromised patients.6 Common acute adverse effects associated with candidate SARS-CoV-2 vaccines reported to date include low-grade fever, myalgias, headache, nausea, fatigue, and soreness/redness at the injection site. These acute adverse effects were more pronounced after the booster dose (2nd vaccine dose) in some of the trials. Long-term adverse effects have not been defined for SARS-CoV-2 vaccines and will be available once phase III trials have completed long term follow up in healthy volunteers. A national monitoring system exists for reporting vaccine-related adverse events.
The efficacy of protein-based or inactivated (killed) SARS-CoV-2 vaccines in immunocompromised patients has yet to be studied. Prior experience with inactivated or killed virus vaccines have demonstrated some efficacy in immunocompromised patients, leading some societies to recommend vaccination of this population.7 Vaccine responses are influenced by the underlying disease and the type and timing of recent therapy. The adjuvanted recombinant (protein-based) zoster vaccine was safe and elicited robust humoral and cellular immune responses across patients with hematologic malignancies when administered during or up to six months after immunosuppressive therapy. However, response rates in patients with chronic lymphocytic leukemia (CLL) or non-Hodgkin lymphomas, many likely treated with anti-CD20 containing regimens, were lower than in the rest of the hematologic malignancies.8
Flu vaccines using killed virus are safe and can elicit humoral immune responses in immunocompromised patients, but response rates appear to be highly variable, reported between 15 and 63 percent of CLL patients not actively treated, and in 7 to 26 percent in patients on BTK inhibitor (ibrutinib) therapy.
The immune response to varicella zoster is a memory response, as the vast majority of people have formed antibodies against varicella zoster virus in childhood. Similarly, response to influenza is, at least in part, a memory response. In contrast, response to SARS-CoV-2 will require a de novo immune response and much less is known about how well immunocompromised patients will be able to generate such a response.
What is known about the safety and efficacy of attenuated live vaccines in immunocompromised patients?
Live attenuated vaccines carry the risk of converting to pathogenic strains with particular risk in immunocompromised patients. It is unclear if a live attenuated SARS-COV-2 vaccine will have the same major risk, but due to the theoretical concerns, a live vaccine should be avoided in immunocompromised patients. Another potential risk from live-attenuated vaccines in general is the possible transmission of the virus to close contacts of the vaccinees. The only live SARS-CoV-2 vaccines are in production in India and Turkey; the COVID-19 vaccines currently available in the United States are not live vaccines.
What is known about the safety and efficacy of the mRNA vaccines in immunocompromised patients?
Pfizer’s BNT162b2 and Moderna’s mRNA-1273 mRNA vaccines have been available in the United States since December 2020 after the FDA granted the vaccines EUA status. Since then, safety data regarding the two mRNA vaccines during the vaccination roll outs have been released. Based on the phase II/III clinical trials of the vaccines, the most common adverse effects include pain at the injection site, fever, headache, fatigue, and joint pain. A rare adverse effect reported is Bell’s palsy; both BioNTech/Pfizer and Moderna reported four and three cases, respectively, of Bell’s palsy in the vaccine recipients who participated in their phase III trials, respectively.1,2 Anaphylaxis due to vaccination with either the Pfizer or Moderna vaccine has been reported at a rate of 4.7 cases/million doses and 2.5 cases/million doses, respectively.9
Both the BNT162b2 and the mRNA-1273 COVID-19 vaccines have been associated with an increased risk of myocarditis after mass distribution of the vaccines. The incidence has been low, with one study reporting a rate of 1.4 and 4.2 per 100,000 vaccinated individuals within 28 days of vaccination with the BNT162b2 and mRNA-1273 COVID-19 vaccines, respectively.10 Conversely, COVID-19 has also been associated with the development of cardiac toxicity including myocarditis, though data are limited. Many of these patients presented with cardiogenic shock and progressed to death based on data from systemic reviews.11 Due to the low incidence of myocarditis after vaccination and high probability of severe COVID-19 in unvaccinated individuals, patients are strongly encouraged to undergo vaccination.
In immunocompromised patients, data are limited, but some reports have been published for cancer patients and solid organ transplant recipients 12-15; vaccine efficacy ranged from 43 percent to 95 percent after the second dose of the mRNA vaccine based on antibody assays. In cancer patients, side effects were mostly mild and included injection site pain, flu-like symptoms, fever, and headaches; similar findings were seen in solid organ transplant recipients. Data regarding safety in hematopoietic cell transplant recipients are beginning to emerge.
What is known about the safety and efficacy of the adenoviral vector vaccines in immunocompromised patients?
The only adenovirus vector–based vaccine approved under EUA in the Unite States is Johnson & Johnson/Jansenn’s Ad26.COV2.S vaccine. The vaccine received EUA from the FDA on Feb 27, 2021, and has the least experience in the general public. Like the mRNA vaccines, the most common adverse effects were pain at the injection site, headaches, fatigue, muscle pain, nausea, and fever.3 Two cases of anaphylaxis were reported to the FDA after the vaccine received EUA. There are limited data regarding safety in immunocompromised patients.
Numerical imbalances were noted in the phase III clinical trial for certain unsolicited adverse effects such as thromboembolic events, seizures, and tinnitus.3 Since receiving approval and undergoing widespread distribution, more cases of atypical clotting were reported to the FDA. After six cases of cerebral venous sinus thrombosis were reported to the FDA, administration and distribution of this vaccine were halted in the United States on April 13, 2021. On April 23, the CDC and FDA made a joint announcement to resume distribution of the Johnsons & Johnson/Janssen SARS-CoV-2 vaccine after determination that the incidence of thrombosis is very low. A new warning was added for rare clotting events in women between the ages of 18 and 49. Individuals who report dizziness, headache, or other neurological symptoms that may suggest a sinus vein thrombosis or symptoms in accordance with other unusual thrombotic locations should undergo further medical evaluation to diagnose or rule out thrombotic events. It is not clear if the rate of clotting would be different in immunocompromised patients.
Are any trials of SARS-CoV-2 vaccines being done in immunocompromised populations?
Most SARS-CoV-2 phase II/III vaccine trials required patients to be off immunosuppression for a certain period to be eligible. This may not be feasible in patients who are receiving therapy for solid organ transplantation, graft-versus-host disease, or hematologic malignancy. It is unclear how the different SARS-CoV-2 vaccine candidates will specifically affect different forms of immune abnormalities. Given the diversity of various immunocompromised patient populations, it is possible that candidate SARS-CoV-2 vaccines may differ in their efficacy and safety for these patients. Clinical trials for immunocompromised patients are ongoing.
If immunocompromised patients were not included in the vaccine trials and are less likely to respond to a SARS-CoV-2 vaccine, should they still receive it? What is the timing in relation to chemotherapy, transplant, antibody therapy, splenectomy, etc.? Should higher vaccine doses or multiple vaccine types be used?
A full discussion of vaccination in patients undergoing stem-cell transplantation or CAR-T therapy is available in separate FAQs. The risks and benefits for immunocompromised patients receiving a SARS-CoV-2 vaccine should be weighed on a case-by-case basis, with consideration of the incidence of infection in the community. This will depend on the approved vaccine formulation available, level of immunosuppression the patient has received, and the underlying reason for immunosuppressive therapy (e.g., cancer treatment, transplantation). If plans to proceed with the SARS-CoV-2 vaccine are made, vaccination is recommended at least two to four weeks prior to the planned immunosuppressive therapy, transplant, or splenectomy. If the patient is receiving or has received immunosuppressive therapy, consider vaccination six months after the patient has been taken off therapy to increase the likelihood of developing immunity (see potential laboratory testing). After hematopoietic cell transplantation, inactivated vaccines have generally shown low incremental risks and have not caused or worsened graft-versus-host disease; thus, inactivated vaccines are generally started after three to six months. If SARS-CoV-2 infection rates are low in a community and a given patient is expected to have improved immune status in upcoming months, clinical judgment is appropriate when weighing the desire for protection as early as possible versus delaying vaccination to give the best chance for response. These recommendations may change, based on the results of the approved vaccine trials. Most experts recommend vaccination if the vaccine is safe for use, even if the expected protection rate is lower than the general population as a partial response may mitigate severe outcomes from COVID-19.
Importantly, vaccination does not change required precautionary behaviors such as masking, social distancing, and frequent hand hygiene. Influenza vaccination should also be administered to immunocompromised patients to reduce the burden of influenza infection and possible dual infection with SARS-CoV-2. Finally, all healthcare workers and household contacts should receive a SARS-CoV-2 vaccine when available to help protect immunocompromised patients, like the recommendations for influenza.
Whether or not an immunocompromised patient is known to have been previously infected with SARS-CoV-2 should not affect the decision of whether to vaccinate. Although some immunity is anticipated from experiencing a COVID-19 clinical infection, this immunity may be insufficient or wane over a short period of time, especially in immunocompromised hosts. However, increased adverse effects could be seen with vaccination, like what is observed with the second dose in a two-dose vaccine series.
Until more is known, different SARS-CoV-2 vaccines are not recommended to the same patient. Although measuring titers may eventually be helpful to assess response, more information is needed. Giving more inoculations than what is recommended at the present time or administering higher doses of an approved SARS-CoV-2 vaccine is highly discouraged at this time.
References
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403-416.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603-2615.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187-2201.
- Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467-478.
- Zhu FC, Guan XH, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479-488.
- Kamar N, Abravanel F, Marion O, et al. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385(7):661-2.
- Bos R, Rutten L, van der Lubbe JEM, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91.
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019;19(9):988-1000.
- Shimabukuro TT, Cole M, Su JR. Reports of Anaphylaxis After Receipt of mRNA COVID-19 Vaccines in the US-December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101-1102.
- Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021;375:e068665.
- Sawalha K, Abozenah M, Kadado AJ, et al. Systematic Review of COVID-19 Related Myocarditis: Insights on Management and Outcome. Cardiovasc Revasc Med. 2021;23:107-113.
- Ou MT, Boyarsky BJ, Motter JD, et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation. 2021.
- Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the First Dose of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation. 2021;105(5):e56-e57.
- Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765-778.
- Waissengrin B, Agbarya A, Safadi E, et al. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22(5):581-583.