ASH-ASTCT COVID-19 Vaccination for HCT and CAR T Cell Recipients: Frequently Asked Questions
While we regularly review and update the information provided here, this FAQ reflects only the best information available at a given point in time. The COVID-19 pandemic is a rapidly evolving global health crisis and new information may have become available since the revision date for each published FAQ.
(Version 5.0; last updated March 22, 2022)
Input from Fareed Khawaja, MD; Roy F. Chemaly, MD, MPH; Sanjeet Dadwal, MD; Steven A. Pergam, MD, MPH; John Wingard, MD; Jeffery Auletta, MD; Zeinab El Boghdadly, MBBCh; Maheen Abidi, MD; Alpana Waghmare, MD; Zainab Shahid, MD; Laura Michaels, MD; Joshua Hill, MD; Tobias M. Hohl, MD, PhD; Mini Kamboj, MD; Michael Boeckh, MD, PhD; and Genovefa Papanicolaou, MD.
Introduction
Vaccination recommendations have been attuned since the first COVID-19 vaccines became available. The changes reflect the access to new data; the need to optimize vaccination uptake and timeline during new surges and the spread of new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); and the need to better protect vulnerable populations. Our understanding of vaccine responses in immunocompromised patients has improved, leading to revised schedules and changes in the number of doses needed. Advances in therapeutics such as monoclonal antibodies for prevention and treatment of COVID-19 have become available and can be given irrespective of vaccination status. However, COVID-19 vaccines remain the cornerstone for prevention of severe illness, hospitalization, and death from SARS-CoV-2. The American Society of Transplantation and Cellular Therapy (ASTCT), the National Marrow Donor Program (NMDP), and the American Society of Hematology (ASH) have continued to engage with the Centers for Disease Control and Prevention (CDC) to better understand, communicate, and implement these changes.
Summary of changes to this FAQ:
- Updated vaccination schedule for immunocompromised patients in alignment with the new recommendations from CDC for this specific population
- Updated recommendations regarding vaccination timing in patients who have received monoclonal antibodies for prevention or treatment of COVID-19
Introduction
The SARS-CoV-2 pandemic continues to cause excess morbidity and mortality in the United States and worldwide. Hematopoietic cell transplant (HCT) and chimeric antigen receptor T (CAR T) cell recipients are at higher risk for serious complications from the virus, including hospitalization, intensive care unit admission, and death from COVID-19. These patients also face other comorbidities associated with COVID-19–related mortality, including older age, cardiovascular disease, renal dysfunction, and high-level immunosuppression, among many others that further deepen and drive worse outcomes.
In the United States, two novel messenger RNA (mRNA) vaccines and one novel adenovirus vector–based vaccine have been either formally approved by the U.S. Food and Drug Administration (FDA) or are approved under the FDA’s Emergency Use Authorization (EUA). The BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 (Moderna) COVID-19 vaccines have both been shown in large phase III clinical trials to be more than 90 percent effective at preventing lab-confirmed COVID-19 illness and severe infections. The single-dose recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector-based vaccine (Ad26.COV2.S; Johnson & Johnson/Janssen) reduced the incidence of symptomatic COVID-19 with a reported efficacy of 66.1 percent (72% in the United States) based on data from the phase III clinical trial. The overall lower efficacy was thought to be due to the newly emerging SARS-CoV-2 variant arising from South Africa (20H/501Y.V2 variant [B.1.351]), which was the predominant strain circulating in South Africa at the time of the clinical trial, and accounted for 95 percent of the sequenced isolates.
Responses to COVID-19 vaccines are likely to be blunted in HCT, or CAR T cell recipients compared with healthy individuals. However, despite the scarcity of data, the high level of protection afforded to those vaccinated in the clinical trials, and the overall safety of the vaccine in clinical trials and post-EUA experience, ASTCT and ASH strongly support vaccination of this vulnerable patient population, along with their caregivers, family, and household contacts.
This FAQ will be updated periodically as new data become available. All current guidance and responses are based on opinions of the ASTCT/ASH COVID-19 Vaccine expert panel.
Table. Recommended COVID-19 Vaccination Schedule for Individuals With Moderate or Severe Immunocompromised Conditions
| Primary Vaccination | Age Group | No. of Primary Vaccine Doses | No. of Booster Doses | Interval Between 1st and 2nd Dose | Interval Between 2nd and 3rd Dose | Interval Between 3rd and 4th Dose |
| Pfizer-BioNTech | 5–11 years | 3 | NA | 3 weeks | ≥4 weeks | N/A |
| Pfizer-BioNTech | ≥12 years | 3 | 1 | 3 weeks | ≥4 weeks | ≥3 months |
| Moderna | ≥18 years | 3 | 1 | 4 weeks | ≥4 weeks | ≥3 months |
| Janssen | ≥18 years | 1 Janssen, followed by 1 mRNA | 1 | 4 weeks | ≥2 months | N/A |
Source: “Use of COVID-19 Vaccines in the United States”
SECTION A: RECOMMENDATIONS ON TIMING OF COVID-19 VACCINE IN HCT AND CAR T-CELL RECIPIENTS, AND CONSIDERATIONS FOR DELAY
When is the recommended time to administer the available COVID-19 vaccines to autologous HCT, allogeneic HCT, and CAR T-cell recipients?
HCT or CAR T cell recipients are often immunosuppressed for months afterward due to conditioning regimens, maintenance therapies, immunosuppressive drugs, hypogammaglobinemia, or development of graft-versus-host disease (GvHD, in allogeneic HCT recipients); these factors may lead to a blunted immune response and affect vaccine efficacy. Yet by delaying immunizations, these patients are at risk of severe and life-threatening COVID-19 if they acquire the infection. Based on prior antigen-based vaccine trials in allogeneic HCT recipients, initiating vaccination series three months versus six months after transplantation did not affect induction of immunogenicity. Clinical trial data to determine the optimal time to initiate vaccinations in HCT and CAR T cell recipients is unfortunately lacking but is of high priority. Retrospective studies have demonstrated variable response in HCT recipients. One potential concern is the efficacy of the Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine in patients with prior adenovirus infection. This was noted with the use of recombinant adenovirus serotype 5 (Ad5). As Ad26 does not commonly circulate in the general population, pre-existing antibodies to this strain are unlikely. It was also reported in the phase I trial for Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine that levels of Ad26 neutralizing antibodies did not correlate with vaccine efficacy. On another note, the different currently available COVID-19 vaccines were not evaluated head-to-head with each other, making it improper to compare vaccine effectiveness based only on phase III trial data that compared each vaccine to a placebo.
Based on the current evidence of high efficacy and safety in the general patient population, including individuals with underlying conditions, the current mRNA SARS-CoV-2 vaccines could be offered as early as three months post-transplantation to HCT and CAR T cell recipients to prevent infection and severe disease, though efficacy may not be optimal as suggested in situations of influenza community outbreaks. In most situations, Pfizer-BioNTech or Moderna COVID-19 Vaccines are preferred over the Johnson & Johnson/Janssen COVID-19 Vaccine for primary and booster vaccination.
When should delay of vaccination be considered in HCT or CAR T-cell recipients?
Cytotoxic or B-cell–depleting therapies after HCT or CAR T-cell therapy are often used for maintenance therapy, which may contribute to poor vaccine immune response. Patients scheduled for such therapy should complete their SARS-CoV-2 vaccination when feasible prior to initiation or between cycles of cytotoxic or B-cell–depleting therapies if possible. Based on a phase I trial of the mRNA SARS-CoV-2 vaccines, peak neutralizing antibodies developed seven to 14 days after the second dose of the vaccine series in patients without prior infection. Similarly, a rise in neutralizing antibodies was seen 15 days after a single-dose recombinant, replication-incompetent Ad26 vector-based vaccine in phase I studies. HCT and CAR T recipients scheduled to undergo cytotoxic or B-cell–depleting therapies could be offered the COVID-19 vaccine prior to therapy and allowed at least two weeks to pass after the second dose to allow memory T cell formation prior to giving cytotoxic or B-cell–depleting therapies if feasible.
Human intravenous immunoglobulins (IVIGs) are often given to patients with hypogammaglobinemia due to poor B-cell function. As SARS-CoV-2 becomes more widespread, immunoglobulins to SARS-CoV-2 may be detectable in pooled IVIG. Theoretically, the immunoglobulins would mask the antigens and dampen the immune response to the vaccines and cross react with serologic testing; for this reason, IVIG recipients were excluded from the phase III mRNA COVID-19 vaccine trials. However, based on the CDC recommendations, no delay in vaccination is recommended for patients who are receiving IVIG. These recommendations may change when more data are available.
What is the currently recommended vaccination schedule for HCT or CAR T cell recipients?
We recommend the primary vaccination and booster schedule approved by the FDA and recommended by CDC/Advisory Committee on Immunization Practices (ACIP) for immunocompromised patients (Table 2). The CDC now considers the primary series for immunocompromised patients to consist of 3 mRNA doses or 1 dose of the adenovirus vector-based vaccine followed by a second dose of the mRNA vaccine. All fourth doses after an mRNA series or third dose after the adenovirus vector-based vaccine series are considered boosters.
Table 2. REVISED COVID-19 Vaccination Schedule for People Who Are Moderately or Severely Immunocompromised
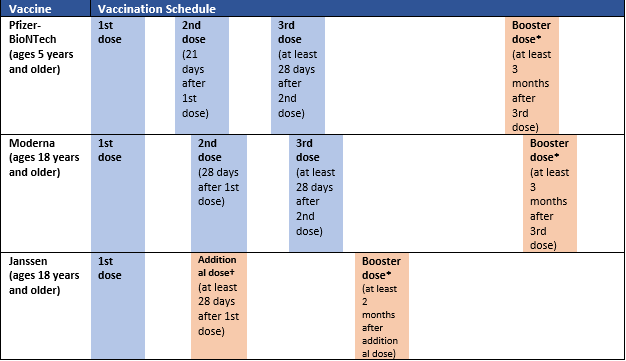
*Any COVID-19 vaccine can be used for the booster dose in people ages 18 years and older, though mRNA vaccines are preferred. For people ages 12-17 years, only Pfizer-BioNTech can be used. People ages 5-11 years should not receive a booster dose.
†Only Pfizer-BioNTech or Moderna COVID-19 vaccines should be used.
From Hall E. Updates to Interim Clinical Considerations for Use of COVID-19 Vaccines. Available at: https://stacks.cdc.gov/view/cdc/114161. Last accessed: March 25, 2022.
After the receipt of the original series of either of the two mRNA vaccines, a third dose is recommended 28 days or more after the second dose. Furthermore, the time between the third dose and the booster dose (the 4th dose) was reduced from five to three months for recipients of the mRNA vaccines. This change was not examined in HCT or CAR T cell populations. Patients who received the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) are eligible to receive a second dose of the mRNA vaccine 28 days or more after the first dose, followed by a booster dose 2 months after the second dose. The booster can be either the Ad26.COV2.S vaccine (Johnson & Johnson/Janssen) or the mRNA vaccine. These recommendations were based on waning protective antibodies over time in immunocompromised individuals, including transplant recipients, and the concern that without a third and fourth dose, these patients would be at high risk for severe COVID-19.
Should we revaccinate HCT or CAR T cell recipients regardless of whether they were partially or fully vaccinated prior to transplantation or cellular therapy?
In patients who underwent COVID-19 vaccination prior to HCT or CAR T-cell therapy, there is major concern for loss of immunity. Despite the lack of data on COVID-19 vaccines, we can extrapolate from prior experience with other preventable infections post-transplantation to predict the loss in immunity after HCT. It has already been demonstrated that the protection conferred by childhood vaccinations, such as measles, mumps, and rubella, are often not retained post-transplantation, necessitating the need for revaccination post-transplantation. Multiple professional societies recommend repeating all vaccinations post-transplantation, regardless of a patient’s vaccination status prior to transplantation. The ASTCT, Center for International Blood and Marrow Transplant Research (CIBMTR) and the National Marrow Donor Program (NMDP) strongly recommend SARS-CoV-2 revaccination following HCT or CAR T-cell therapy. This is in alignment with CDC/ACIP recommendations.
Recipients of HCT or CAR T-cell therapy who received one or more doses of COVID-19 vaccine prior to or during treatment should be revaccinated (i.e., complete primary vaccination and any recommended additional or booster doses). See Table 1 for vaccination interval in immunocompromised patients.
An mRNA vaccine is preferred for revaccination, regardless of vaccine administered prior to transplantation or administration of cellular products.
Revaccination should start at least three months (12 weeks) after transplantation or CAR T-cell therapy; however, the patient’s clinical team is best positioned to determine the timing based on degree of immune compromise, need for revaccination, and appropriate timing of revaccination.
Thus, we recommend repeating the COVID-19 vaccination series (which is primary series of 3 doses as described above and the 4th booster dose) at least three months after HCT or CAR T-cell therapy, regardless of vaccination status prior to transplantation or cellular therapy. Repeating the vaccination series with the mRNA vaccine is recommended, but with some exceptions such as access non-mRNA vaccine may be used.
When should HCT and CAR T cell recipients receive an additional dose of the COVD-19 vaccine if they become infected with SARS-CoV-2 between doses?
If COVID-19 vaccinees become infected prior to the second dose, the CDC recommends delaying the second dose of either the BNT162b2 (Pfizer/BioNTech) or the mRNA-1273 (Moderna) COVID-19 vaccine series until the symptoms have resolved and isolation precautions are discontinued. Based on data from patients previously infected with COVID-19 prior to mRNA vaccination series, HCT and CAR T cell recipients with COVID-19 between doses could resume vaccination of their respective vaccines once symptoms have resolved and isolation precautions are discontinued, as there is no indication so far of vaccine-associated enhanced disease (VAED) or other serious adverse events.
When can the current COVID-19 vaccines be given after therapy with SARS-CoV-2 monoclonal antibodies or convalescent plasma in HCT and CAR T cell recipients?
There are limited published safety and efficacy data on the use of mRNA SARS-CoV-2 vaccines after receipt of SARS-CoV-2 monoclonal antibodies or convalescent plasma in patients as part of their COVID-19 treatment; these patients were specifically excluded from the phase III mRNA COVID-19 vaccine trials. Despite early reports that monoclonal antibodies may reduce serologic responses from vaccines, there is no clear clinical benefit in delaying vaccination due to recent receipt of monoclonal antibodies. Based on the CDC recommendations, COVID-19 vaccination should not be deferred after receipt of convalescent plasma or monoclonal antibodies directed at SARS CoV-2 for postexposure prophylaxis or treatment. Conversely, due to the restrictions from the EUA for Evusheld (tixagevimab/cilgavimab), administration of Evusheld should be delayed for two weeks after vaccine administration.
Can SARS-CoV-2 monoclonal antibodies be given to HCT and CAR T cell recipients who develop COVID-19 after receipt of mRNA COVID-19 vaccines?
Patients who are exposed to or develop SARS-CoV-2 infection after receiving the COVID-19 vaccine, are eligible for monoclonal antibodies that retain neutralizing activity against the circulating variant(s) for postexposure prophylaxis or treatment of COVID-19.
SECTION B: COVID-19 VACCINE SAFETY IN HCT AND CAR T-CELL RECIPIENTS
Has the mRNA SARS-CoV-2 and recombinant adenovirus vaccines platform previously been investigated in the immunocompromised patient population?
While there are no other licensed mRNA vaccines in the United States, mRNA-vaccine platforms have been studied in the treatment of cancer and other infections such as influenza, Zika, rabies, and cytomegalovirus. With the ongoing mRNA SARS-CoV-2 vaccine uptake, data in immunocompromised patients became available. One study involving cancer patients with either solid tumors or hematologic malignancies demonstrated poor antibody response after a single dose of the Pfizer mRNA vaccine. A more pronounced antibody response was seen after the second dose in patients with solid tumors. Another study from the University of Pittsburgh showed that 46 percent of patients with hematologic malignancies did not produce antibodies after two doses of the mRNA vaccines. Similar results were described in a study of solid organ transplant recipients. Despite the suboptimal antibody responses in this immunocompromised population, no major safety events were reported after the use of mRNA vaccines. These studies did not report clinical outcomes of the vaccinated patients and were unable to correlate vaccination with reduced risk of COVID-19.
While adenoviral vectors have been tested in far more people than the mRNA vaccines prior to COVID-19, no adenoviral vector vaccines have demonstrated prevention of diseases in humans, nor are any licensed for use in the United States. There are limited data regarding adenovirus vector–based vaccines in immunocompromised patients. Further investigation is warranted to study the immunogenicity and durability of protection from these vaccines among this population. The adenovirus vector (Ad26) used in the Janssen vaccine is replication incompetent and should not pose a safety concern for immunocompromised hosts.
As previously mentioned, we strongly recommend reporting any suspected adverse events in immunocompromised patients through the vaccine adverse events reporting system (VAERS) (https://vaers.hhs.gov/reportevent.html).
What is known about the safety of mRNA SARS-CoV-2 vaccines?
The mRNA SARS-CoV-2 vaccines were administered to nearly 70,000 study participants, and safety profile at two months median follow-up has not raised any significant concerns. HCT and CAR T cell recipients were excluded from these trials; however, individuals with well-controlled HIV infection and CD4>350 were included. Similar to other vaccines, short-term adverse effects included local injection site reactions, fever, fatigue, and headache, and they typically resolved within one to two days. Adults older than 55 years experienced decreased frequency and severity of local injection site reactions and systemic adverse effects. Serious adverse effects were seen in 0.5 to 1.5 percent of study participants across the three reported trials with similar distribution in control and vaccine arms.
Both the BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 (Moderna) COVID-19 vaccines have been associated with an increased risk of myocarditis after mass distribution of the vaccines. The incidence has been low, with one study reporting a rate of 1.4 and 4.2 per 100 000 vaccinated individuals within 28 days of vaccination with the BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 (Moderna) COVID-19 vaccines, respectively. On the other hand, COVID-19 has also been associated with the development of cardiac toxicity including myocarditis, though the data are limited. Many of these patients presented with cardiogenic shock and progressed to death based on data from systemic reviews. Due to the low rate of occurrence after vaccination and high probability of severe COVID-19 in unvaccinated individuals, patients are strongly encouraged to undergo vaccination.
Although extrapolation of safety data in the HCT and CAR T cell recipients can be challenging, significant adverse effects beyond the early postvaccination period are not anticipated, and the benefits from vaccines may outweigh any short-term or long-term adverse effects. Close monitoring for early and late post-vaccination effects is warranted. Any adverse events should be reported to the vaccine adverse events reporting system (VAERS) and is strongly recommended in immunocompromised patients (https://vaers.hhs.gov/reportevent.html).
What is known about the safety of the recombinant adenovirus vector SARS-CoV-2 vaccine?
The three recombinant adenovirus vector vaccines in clinical trials make use of different adenovirus serotypes: the Ad5-nCoV (CanSino) vaccine uses the human-derived serotype 5 (Ad5), the ChAdOx1 (AstraZeneca) vaccine uses the chimpanzee-derived serotype AZD1222, and the AD26.COV2.S (Johnson & Johnson/Janssen) vaccine uses human-derived Ad26. To date, only AD26.COV2.S has received EUA by the FDA. Provided information is limited to the AD26.COV2.S vaccine.
A total of 44,325 people were enrolled onto the phase III trial for AD26.COV2.S from eight different countries, including the United States. Of those, 22,174 received the vaccine. Patients with controlled HIV were included as well, but a separate analysis of this population was not released. Like the mRNA vaccines, the most common adverse effects were pain at the injection site, headaches, fatigue, muscle pain, nausea, and fever. Serious adverse effects were seen in 0.7 percent of individuals who received the vaccine. A hypersensitivity event was reported in one case, and anaphylaxis in two cases, to the FDA. The FDA fact sheet also notes that the vaccine may have lower efficacy in immunocompromised patients, but no data are cited. Additionally, numerical imbalances were noted for certain unsolicited adverse effects such as thromboembolic events, seizures, and tinnitus. Please see below for more details regarding thrombosis associated with recombinant adenovirus vaccines. It is challenging to extrapolate safety to HCT and CAR T cell recipients from the available data, and prior to administration of this vaccine, potential risks and benefits should be weighed with shared decision-making with the patient. Close monitoring for early and late postvaccination adverse effects is warranted.
What is the safety of mRNA and recombinant adenovirus vector SARS-CoV-2 vaccines in patients with unknown prior SARS-CoV-2 exposure?
Based on prior studies in severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome (MERS), there is a theoretical concern that formation of low titer neutralizing antibodies that can precipitate a VAED. In the registration trials of these vaccines, there were no indications of VAED among the study population enrolled (which lacked HCT or CAR T cell recipients) and included a small number of patients with history of cancer (<3%) and 1,218 individuals with controlled HIV. These trials included only a subset of study participants who were seropositive for SARS-CoV-2 at time of study entry (9.6% had evidence of previous infection) and participants who developed COVID-19 in the vaccine arm.
What are the risks of serious allergic reactions from mRNA and recombinant adenovirus vector SARS-CoV-2 vaccines?
For individuals with a history of anaphylaxis to other vaccines, counselling for a potential similar reaction is recommended and should be monitored for 30 minutes if vaccinated. All individuals who receive the vaccine need to be monitored on site immediately following vaccination for at least 15 minutes. It is still recommended for individuals with drug or food allergies to receive the SARS-CoV-2 vaccine. The potential for anaphylaxis to either mRNA vaccine is 2.5 to 4.7 cases per million doses.
The risk of anaphylaxis reported after the Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine is extremely low. The only contraindication to this vaccine is an immediate severe allergic reaction to one of the components of the Ad26.COV2.S (Johnson & Johnson/Janssen) or known allergy to polysorbate. Individuals with history of anaphylaxis to other vaccines, drugs, or foods can safely receive the vaccine with close monitoring. Patients who are allergic to ingredients in the mRNA vaccines or those with a known allergy to polyethylene glycol should consider getting the recombinant adenovirus vector SARS-CoV-2 vaccine or AD26.COV2.S, and vice versa. The CDC also recommends that those who cannot get the second dose of the mRNA SARS-CoV-2 vaccine due to contraindications (such as allergic reaction to the first dose) may be given a single-dose recombinant adenovirus vector SARS-CoV-2 vaccine at or 28 days after the first dose. The CDC website provides detailed guidance on vaccine ingredients and triaging candidates based on their history of allergic reactions.
Is it safe to combine routine post-transplant vaccines with SARS-CoV-2 vaccines?
Routine post-transplant vaccines can be given concomitantly with COVID-19 vaccines.
Is it safe to use COVID-19 vaccines for treatment of an acute COVID-19 in HCT and CAR T-cell recipients?
Although data from vaccine clinical trials have demonstrated safety in patients with COVID-19, neither the mRNA SARS-CoV-2 nor the recombinant adenovirus vector vaccines are a replacement for therapy. HCT or CAR T-cell therapy recipients with recent COVID-19 should be offered the vaccine once symptoms resolve.
COVID-19 vaccines should not be used for treatment of COVID-19.
What are some considerations or concerns post–COVID-19 vaccination among HCT and CAR T-cell recipients?
A study in immunocompetent individuals (<56 years of age) showed that COVID-19 vaccine BNT162b1 elicits CD4+ and CD8+ T cell responses, with TH1 cell responses and increased production of interferon γ (IFN-γ), IL-2, and IL-12. Similarly, the phase I data for the recombinant adenovirus vector SARS-CoV-2 vaccine reported an increase in IFN-γ ELISPOT responses, with no IL-4 response, favoring a TH1 cell response. With exclusion of transplant recipients from the vaccine phase II/III trials, it remains unknown whether postvaccination inflammatory reactions could increase risk for GvHD, hemophagocytic lymphohistiocytosis, and transplant-associated thrombotic microangiopathy. Thousands of doses have been administered in HCT and CAR T recipients, and up to now, there are no reports of increased GVHD; however, close monitoring and reporting of such events are strongly recommended.
What are the clotting risks associated with administration of the COVID-19 vaccine, in particular the AZD1222 (AstraZeneca) and AD26.COV2.S (Janssen) vaccines?
Previously, cases of thrombosis at unusual sites (e.g., sinus or cerebral vein thrombosis) and cases of disseminated intravascular coagulation had been observed within four to 16 days after vaccination with the AZD1222 (AstraZeneca) vaccine in countries outside the United States. Affected individuals were mostly women younger than 55 years. Initial reports stated that the vaccine was unlikely linked to these cases; however, updated incidence of atypical clotting was 1 in 100,000 vaccine recipients; some of these events led to death. The mechanisms of these clotting events were similar to heparin-induced thrombocytopenia and thrombosis (HITT) due to the presence of IgG antibodies against platelet factor 4 (PF4). As these thrombotic events occurred in younger individuals, many European countries are now offering this vaccine to older populations. The AZD1222 (AstraZeneca) vaccine is not available in the United States.
Similar thrombotic events were also noted with the AD26.COV2.S vaccine (Johnsons & Johnson/Janssen). Cases of serious thromboembolic events (6 cases of deep venous thrombosis, 4 cases of pulmonary embolism, and 1 case of transverse sinus thrombosis) in the vaccine recipient group were reported in a phase III trial, that were not clearly linked to the vaccine. However, antibodies against PF4 were detected in few cases. After six cases of cerebral venous sinus thrombosis were reported to the FDA, administration and distribution of this vaccine was halted in the United States on April 13, 2021. On April 23, the CDC and FDA made a joint announcement to resume distribution of the Johnsons & Johnson/Janssen SARS-CoV-2 vaccine after determination that the incidence of thrombosis is very low and the benefit of the vaccination outweighed the risk. A new warning was added for rare clotting events in women between the ages of 18 and 49 years. Individuals who report dizziness, headache, or other neurological symptoms that may suggest a sinus vein thrombosis or symptoms in accordance with other unusual thrombotic locations should undergo further medical evaluation to diagnose or rule out thrombotic events.
SECTION C: RECOMMENDATIONS FOR SPECIAL HCT AND CAR T-CELL RECIPIENT POPULATIONS
What additional factors should be considered regarding COVID-19 vaccines for pediatric HCT and CAR T-cell recipients?
In the United States, the age limit for COVID-19 vaccines available under EUA are five years or older for the BNT162b2 (Pfizer) vaccine, and 18 years or older for the mRNA-1273 (Moderna) and Ad26.COV2.S (Johnson & Johnson/Janssen) vaccines. The lower age limit for the BNT162b2 (Pfizer) vaccine administration under the FDA was reduced from 16 to 12 based on phase III trial data, and EUA was granted for patients aged five to 11 years based on phase I data. Moderna has also announced that their trial (TeenCOVE), which enrolled children from the age of 12 to 17 years, has met its endpoint analysis. As in adults, there are no specific data on safety or efficacy available for pediatric HCT and CAR T cell recipients. Recommendations for timing of vaccine administration and revaccinating HCT and CART cell recipients (lower age limit of 5 and 18 years for Pfizer and Moderna, respectively) regardless of their vaccination status prior to transplantation is similar to those in adults. Considerations for vaccination of household contacts, use of serologic assays, use of monoclonal antibodies in the context of vaccination, and co-administration with other vaccines, are the same as in adults. .
Should HCT or CAR T-cell candidates receive the COVID-19 vaccination to prevent severe disease post-HCT or post–CAR T-cell therapy? Should stem cell donors receive the COVID-19 vaccination to prevent disease in transplant recipients?
Currently there is no reported literature on the benefit of COVID-19 vaccination prior to HCT or cellular therapy in preventing severe COVID-19 in HCT or CAR T cell recipients. Based on the time required to complete the vaccine series and the loss of immunity post cellular therapy, this approach to solely prevent potential severe disease is neither feasible nor practical and thus, it is not recommended. Instead, passive immunity can be conferred using long-acting monoclonal antibody that is active against the circulating variant.
Currently, there are no studies demonstrating adoptive transfer of immunity from COVID-19 vaccinated donors to HCT or CAR T cell recipients. Vaccinating stem cell donors prior to stem cell harvesting has not been consistently shown to benefit HCT recipients in prior studies.
Stem cell donors should not be offered the COVID-19 vaccine for the sole purpose of benefiting HCT recipients unless under a research protocol. However, if the donor has been vaccinated, it may be desirable to wait at least two weeks after the second vaccine dose before stem cell donation (if possible) as it may provide some protective effect to the recipient.
How effective are the COVD-19 vaccines in preventing infection from SARS-CoV-2 variants in HCT and CAR T-cell recipients?
SARS-CoV-2 variants have emerged due to the inherent mutagenesis of the virus itself and the continued viral prevalence throughout the United States (CDC Viral Variant Tracker), reflecting low herd immunity. The mRNA COVID-19 vaccine BNT162b2 (Pfizer) effectiveness in preventing COVID-19 against the variants B.1.1.7 and B.1.351 was 89.5 percent and 75.0 percent, respectively, and prevention of severe disease due to these two variants was higher (up to 97.4%). However, the vaccine efficacy against SARS CoV-2 variants was lower than previously reported in the phase III trials and live experience in Israel and the United States. The AD26.COV2.S vaccine was less effective in South Africa and Brazil where the B.1.135 and P.1 variants were widespread, respectively, yet it exceeded the FDA EUA threshold of greater than 50 percent effectiveness in preventing COVID-19 infection. It is not certain how effective the vaccines are in immunocompromised patients.
The latest variant, Omicron (B.1.1.529), with its mutations at the spike protein, has rendered some of the EUA monoclonal antibodies ineffective. The efficacy of the currently approved/EUA COVID-19 vaccines has also been affected by these mutations, leading to breakthrough infections in immunocompetent and immunocompromised individuals. The Omicron variant continues to contest how we can protect our most vulnerable patients considering these major viral mutations. Despite breakthrough infections in vaccinated patients, vaccination provided significant protection from developing severe disease or hospitalization.
Based on serologic testing post COVID-19 vaccination in immunocompromised patients, the current COVID-19 vaccines may not be sufficient in preventing SARS-CoV-2 infection or severe COVID-19 in HCT or CAR T cell recipients. To mitigate this issue, a prophylactic approach to prevent infection or severe disease in patients who are less likely to respond to vaccines is important, such as the use of long-acting monoclonal antibodies that are effective against the circulating variant.
SECTION D: COVID-19 SEROLOGIC TESTING POST VACCINATION IN HCT AND CAR T-CELL RECIPIENTS
What is the appropriate timing and the role of serologic testing for COVID-19 after COVID-19 vaccination?
Neutralizing antibodies against the receptor binding domain of the spike protein are considered protective against reinfection, in contrast to antibodies against the nucleocapsid, which are not thought to be protective. Available vaccines will only produce antibodies to the spike protein. In healthy individuals who had mild to moderate COVID-19 infections, high titers of neutralizing antibodies lasted up to five months after initial infection, with robust antibody response occurring by day 30 post infection. However, the correlation between COVID-19 antibodies and development of subsequent illness is not clear. Similarly, antibody response is expected with COVID-19 vaccination. Durability of response to COVID-19 mRNA-1273 vaccine was assessed in a subset of vaccine recipients. Neutralizing antibody levels were detected in the entire subset at day 119 and 90 days after the first and second dose of the vaccine, respectively. Lower geometric mean titer was observed in vaccine recipients older than 71 years compared with those younger than 70 years. There are limited COVID-19 antibody data in immunocompromised vaccine recipients. In a British study of 56 patients with solid tumor cancers, 44 patients with hematologic malignancies, and 34 healthy controls, anti-S protein was detected 21 days after the first dose of BNT162b2 in 38 percent, 18 percent, and 94 percent of recipients, respectively. Of those, antibody data were available for 25 of the patients with solid tumors and six of those with hematologic malignancies 14 days after the second dose, and anti-S protein was detected in 95 percent and 60 percent, respectively.
However, the antibody response (titer and durability) to the COVID-19 vaccine in HCT and CAR T cell recipients is not known. As the role of serologic testing postvaccination in HCT and CAR T cell recipients is not clear, we do not recommend routine testing with serology unless done under a research protocol.
Conversely, if serologic testing is desired by the patient or health care providers, we recommend testing for SARS-COV-2 antibodies against the spike protein anytime between 30 and 90 days after the second dose of the vaccine. Importantly, some of the commercially available serology assays test for antibodies against the nucleocapsid (N) protein, which are markers of prior natural infection from SARS-CoV-2 and not an indication of immune response to COVID-19 vaccines; thus, understanding which serologic assays are available at your disposal is of utmost importance. Additionally, with increasing prevalence of SARS-CoV-2 infections and vaccination uptake across the United States, pooled IgG may contain antibodies against SARS-CoV-2 spike and nucleocapsid proteins; thus, if serologic testing is desired, we do not recommended testing for SARS-CoV-2 antibodies within four weeks of IVIG or COVID-19–directed monoclonal antibody infusions due to possible false-positive results..
SECTION E: RECOMMENDATIONS FOR THE CLOSE CONTACTS OF HCT AND CAR T-CELL RECIPIENTS REGARDING COVID-19 VACCINATION
Given the lack of published data on the safety and efficacy of the COVID-19 vaccines in immunocompromised patients, what is an effective vaccine strategy to reduce viral transmission to this group of patients?
Viral transmission from COVID-19–positive household contacts poses the highest risk of viral spread to any population, but especially to immunocompromised patients. Other close contacts include health care workers caring for immunocompromised patients, who are also at increased risk for exposure to COVID-19 in the community. Vaccination of household members, close contacts, and health care providers caring for immunocompromised patients is a central strategy to reduce the risk of viral transmission to immunocompromised patients. All close contacts including health care workers are strongly encouraged to get vaccinated if they have access to COVID-19 vaccines.
When should family members, caregivers and/or household contacts who interact with HCT and CAR T-cell recipients be administered COVID-19 vaccines?
Although nosocomial transmission can occur and is associated with higher morbidity and mortality, community exposure is the most common source for many infections among cancer and transplant patients, including COVID-19. With the enhanced focus on infection control efforts in health care settings, including universal masking, social distancing, symptom screening, and frequent SARS-CoV-2 testing for these high-risk patients, hospital and clinic-based transmission is less frequent. However, family members, caregivers, and household contacts are more likely to be the source of SARS-CoV-2 transmission to HCT and CAR T recipients in the context of being unmasked for prolonged periods of time, especially in closed and/or poorly ventilated environments. In a recent meta-analysis of 54 studies with 77,758 participants, the estimated overall household secondary attack rate was 16.6 percent, with higher rates of transmission associated with a symptomatic household member. Models suggest that more than 50 percent of all SARS-CoV-2 infections are a result of transmission from presymptomatic or asymptomatic infections. Therefore, efforts to separate symptomatic contacts from high-risk immunocompromised patients, though still recommended, may not prevent transmission, particularly in home environments. Furthermore, when infected, prolonged viral shedding among immunocompromised patients can potentially put other family members and other close contacts at increased risk. We recommend that all close contacts of HCT and CAR T cell recipients receive COVID-19 vaccines as soon as possible per the CDC recommended vaccination schedule (primary series and boosters).
To date, currently approved/EUA vaccines are known to reduce the severity of COVID-19 disease and its complications, but data on prevention of primary infection or even transmission from those vaccinated have not been adequately demonstrated. Additionally, prior studies have demonstrated the spread of COVID-19 originating from vaccinated household members. For this reason, family members, caregivers, and other household members should continue to wear masks, practicing social distancing and following all current recommendations for preventing SARS-CoV-2 exposure and acquisition.
Is there any foreseeable risk to HCT and CAR T-cell recipients by vaccinating their close contacts with the available or soon-to-be-available COVID-19 vaccines?
Currently, approved mRNA vaccines (Pfizer-BioNTech, Moderna) do not contain live virus; thus, these vaccines are safe to use in close contacts of immunocompromised patients. Similarly, the Johnson & Johnson/Janssen COVID-19 vaccine uses a replication-deficient Ad26 vector that is not transmissible to others. Other candidate vaccines are still in ongoing clinical trials or are under FDA review.
The AstraZeneca-Oxford vaccine consists of live simian adenovirus vector ChAdOx1, containing the full-length structural surface glycoprotein (spike protein) of SARS-CoV-2, but the virus has been modified to be replication-deficient, and it cannot be transmitted to others. This vaccine is currently not approved for use in the United States. The Novavax vaccine candidate (NVX-CoV2373), a protein subunit vaccine delivered with an adjuvant (saponin-based Matrix-M™), is not a live-virus vaccine and is not yet approved for use in the United States. Therefore, when or if these vaccines become available for use in the United States, there is no foreseeable risk of SARS-CoV-2 transmission to immunocompromised patients or their close contacts.
Resources
Coll E, Fernandez-Ruiz M, Sanchez-Alvarez JE, et al. COVID-19 in transplant recipients: The Spanish experience. Am J Transplant 2021;21:1825-37.
Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol 2021;8:e185-e93.
Pinana JL, Martino R, Garcia-Garcia I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol 2020;9:21.
Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in pediatric hematopoietic stem cell transplantation: The experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer 2020;67:e28514.
Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020;383:2603-15.
Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 2020;384:403-16.
Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med 2021.
Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021.
Monin L, Laing AG, Munoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol 2021.
Kamboj M, Shah MK. Vaccination of the Stem Cell Transplant Recipient and the Hematologic Malignancy Patient. Infect Dis Clin North Am 2019;33:593-609.
Cordonnier C, Labopin M, Chesnel V, et al. Randomized study of early versus late immunization with pneumococcal conjugate vaccine after allogeneic stem cell transplantation. Clin Infect Dis 2009;48:1392-401.
Redman RL, Nader S, Zerboni L, et al. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis 1997;176:578-85.
Carpenter PA, Englund JA. How I vaccinate blood and marrow transplant recipients. Blood 2016;127:2824-32.
Machado CM, Cardoso MR, da Rocha IF, Boas LS, Dulley FL, Pannuti CS. The benefit of influenza vaccination after bone marrow transplantation. Bone Marrow Transplant 2005;36:897-900.
Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008;42:637-41.
Zaiss AK, Machado HB, Herschman HR. The influence of innate and pre-existing immunity on adenovirus therapy. J Cell Biochem 2009;108:778-90.
Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med 2021;384:1824-35.
Nazi I, Kelton JG, Larche M, et al. The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 2013;122:1946-53.
Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine 2020;383:2439-50.
Stephenson KE, Le Gars M, Sadoff J, et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021;325:1535-44.
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. New England Journal of Medicine 2021;385:661-2.
Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N Engl J Med 2021.
Ljungman P, Fridell E, Lönqvist B, et al. Efficacy and Safety of Vaccination of Marrow Transplant Recipients with a Live Attenuated Measles, Mumps, and Rubella Vaccine. J Infect Dis 1989;159:610-5.
Cordonnier C, Einarsdottir S, Cesaro S, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). The Lancet Infectious Diseases 2019;19:e200-e12.
Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clinical Infectious Diseases 2013;58:e44-e100.
Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19 https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19.
Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.
Maruggi G, Zhang C, Li J, Ulmer JB, Yu D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol Ther 2019;27:757-72.
John S, Yuzhakov O, Woods A, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 2018;36:1689-99.
Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID-19 mRNA vaccines in hematologic malignancies patients. medRxiv 2021:2021.04.06.21254949.
Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med 2020;383:2427-38.
Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396:467-78.
FACT SHEET FOR HEALTHCARE PROVIDERS ADMINISTERING VACCINE (VACCINATION PROVIDERS). 2021.
Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2020;586:594-9.
Sahin U, Muik A, Derhovanessian E, et al. Publisher Correction: COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature 2021;590:E17.
Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021;325:780-1.
Haynes BF, Corey L, Fernandes P, et al. Prospects for a safe COVID-19 vaccine. Sci Transl Med 2020;12.
Schultz NH, Sorvoll IH, Michelsen AE, et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med 2021.
Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet 2021.
Sadoff J, Davis K, Douoguih M. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination - Response from the Manufacturer. N Engl J Med 2021.
Emergency Use Authorization (EUA) Amendment for an Unapproved Product https://www.fda.gov/media/148542/download.
Pfizer and BioNTech Announce Positive Topline Results From Pivotal Trial of COVID-19 Vaccine in Children 5 to 11 Years https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-positive-topline-results.
Moderna Announces TeenCOVE Study of its COVID-19 Vaccine in Adolescents Meets Primary Endpoint and Plans to Submit Data to Regulators in Early June https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-teencove-study-its-covid-19-vaccine.
Molrine DC, Guinan EC, Antin JH, et al. Haemophilus influenzae type b (HIB)-conjugate immunization before bone marrow harvest in autologous bone marrow transplantation. Bone Marrow Transplant 1996;17:1149-55.
Antin JH, Guinan EC, Avigan D, et al. Protective antibody responses to pneumococcal conjugate vaccine after autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2005;11:213-22.
Winston DJ, Mullane KM, Cornely OA, et al. Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:2116-27.
Address COVID-19 Vaccine Access Challenges with Leadership, Funding, Collaboration and Science https://www.idsociety.org/news--publications-new/articles/2021/address-covid-19-vaccine-access-challenges-with-leadership-funding-collaboration-and-science/.
Meisel R, Kuypers L, Dirksen U, et al. Pneumococcal conjugate vaccine provides early protective antibody responses in children after related and unrelated allogeneic hematopoietic stem cell transplantation. Blood 2007;109:2322-6.
Ambati A, Boas LS, Ljungman P, et al. Evaluation of pretransplant influenza vaccination in hematopoietic SCT: a randomized prospective study. Bone Marrow Transplant 2015;50:858-64.
Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. New England Journal of Medicine 2021.
Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet 2021;397:1819-29.
Addetia A, Crawford KHD, Dingens A, et al. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol 2020;58.
Dispinseri S, Lampasona V, Secchi M, et al. Robust Neutralizing Antibodies to SARS-CoV-2 Develop and Persist in Subjects with Diabetes and COVID-19 Pneumonia. J Clin Endocrinol Metab 2021;106:1472-81.
Widge AT, Rouphael NG, Jackson LA, et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med 2021;384:80-2.
Madewell ZJ, Yang Y, Longini IM, Jr., Halloran ME, Dean NE. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open 2020;3:e2031756.
Selden TM, Berdahl TA. Risk of Severe COVID-19 Among Workers and Their Household Members. JAMA Intern Med 2021;181:120-2.
Elkrief A, Desilets A, Papneja N, et al. High mortality among hospital-acquired COVID-19 infection in patients with cancer: A multicentre observational cohort study. Eur J Cancer 2020;139:181-7.
Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Netw Open 2021;4:e2035057.
Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med 2020;383:2586-8.
Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of Vaccination on Household Transmission of SARS-CoV-2 in England. N Engl J Med 2021;385:759-60.
Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med 2020;383:2320-32.