CAR-T Toxicities Consortium
The Chimeric Antigen Receptor T-Cell (CAR-T) Toxicities Consortium unites investigators from scientific and medical associations, government agencies, and registries, to address emerging toxicities associated with the application of CAR-T therapies.
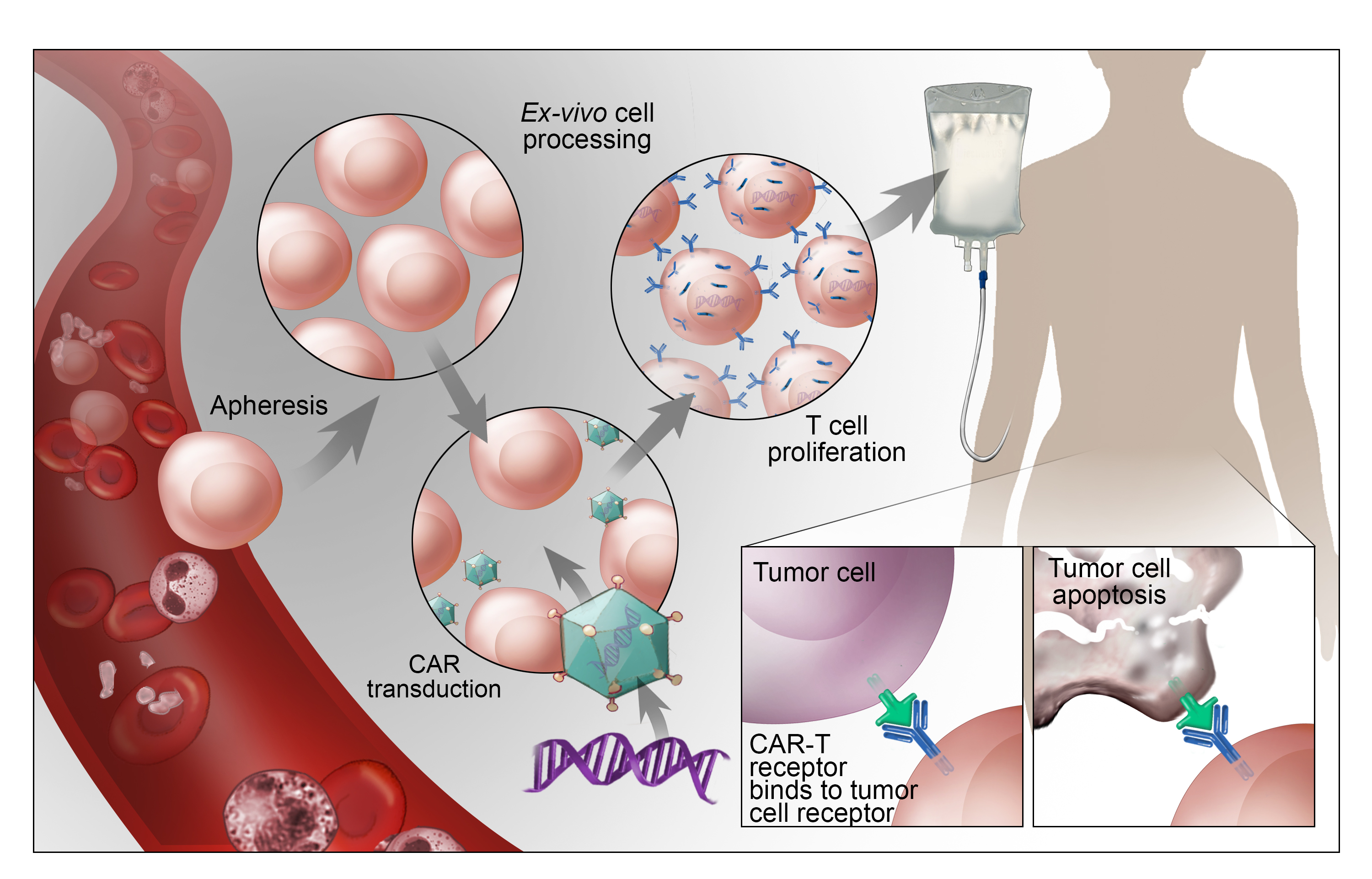
Goals and Objectives
The consortium, consisting of representatives from scientific and medical associations, the U.S. Food and Drug Administration, and the Center for International Blood and Marrow Transplant Research working together, will address urgent needs related to emerging CAR-T adverse events and identify opportunities to address the following areas:
- Increasing investigators’ understanding of the molecular mechanisms surrounding these toxicities and the role conditioning regimens could play in their onset.
- Exploring ways to effectively report and catalog these toxicities to inform future research.
- Developing educational resources for clinicians to inform toxicity management approaches.
- Informing regulatory agencies on the challenges and recent developments in the field surrounding this topic.
Current Focus
The consortium is expected to convene on a bimonthly basis (i.e., every other month), with its first order of business being to conduct a landscape analysis of the field. Based on its assessment, it will then ascertain and develop resources to address important gaps.
Consortium Members
Members consist of individuals who have expertise in CAR T cells and the ability to carry forward recommendations from this consortium to their respective organizations.
American Society of Hematology
Nirali N. Shah, MD, MHSc (Chair)
Robert S Negrin, MD, PhD (Vice Chair)
Alice Kuaban, MS (Staff Liason)
American Association for Cancer Research
Helen E. Heslop, MD, DSc (Hon)
Nikhil C. Munshi, MD
American College of Rheumatology
Maximilian F. Konig, MD, DSc (Hon)
C. Thomas Appleton, MD, PhD
American Society of Gene & Cell Therapy
Isabelle Riviere, PhD
Marco Davila, MD, PhD
ASH Research Collaborative
Saad Usmani, MD, MBA
William Wood, MD, MPH
American Society for Transplantation and Cellular Therapy
Frederick Locke, MD
Marcela Maus, MD, PhD
Center for International Blood and Marrow Transplant Research
Marcelo Pasquini, MD, MS
Society for Immunotherapy of
Cancer
Kristen Hege, MD
Sarah Warren, PhD
U.S. Food and Drug Administration
Nicole Verdun, MD
International Society for Cell & Gene Therapy
Bruce Levine, PhD
Jaap Jan Boelens, MD, PhD
Questions?
For more information on the CAR-T Toxicities Consortium, please contact ASH Director of Scientific Affairs, Alice Kuaban, MS at [email protected].