New Studies Highlight How Immunotherapies Are Transforming Care for Blood Cancers
We updated this press release on December 14, 2021, to include a clarification on a trial endpoint. Under the write-up for abstract 463, please see paragraph six.
Researchers examine opportunities for immune-modulating approaches to improve outcomes and reduce side effects
(WASHINGTON, Dec. 11, 2021) – Four studies being presented during the 63rd American Society of Hematology (ASH) Annual Meeting and Exposition point to the transformative role immunotherapies are playing in the treatment of major blood cancers.
The studies represent a range of approaches that harness patients’ own immune systems to seek and destroy cancer cells. “These studies highlight major areas of investigation that are ongoing in cancer care and treatment,” said press briefing moderator Laurie Sehn, MD, of British Columbia Cancer Centre for Lymphoid Cancer and The University of British Columbia. “They will certainly garner a lot of interest and, in some cases, will likely transform practice.”
Three of the studies focus on non-Hodgkin lymphomas, which affect white blood cells, and one focuses on multiple myeloma, which affects plasma cells. All four studies reflect strategies to use the immune system to fight cancer, although they operate through different mechanisms.
The first study, a phase III trial for treating multiple myeloma, bolsters the current three-drug standard of care with a fourth drug, a monoclonal antibody that is designed to directly fight cancer while also stimulating the immune system. The second study, a phase I/II trial for follicular lymphoma, demonstrates a different approach in which a drug, delivered intravenously, helps a patient’s immune cells recognize and target lymphoma cells, boosting the natural immune response.
The final two studies are phase III trials involving chimeric antigen receptor (CAR) T-cell therapies for large B-cell lymphoma. In this type of therapy, a patient’s T cells are removed, modified, and then infused back into the body, where they find and destroy cancer cells. The studies demonstrate the promise of this approach as a second-line treatment, suggesting an opportunity to consider incorporating these treatments at an earlier stage than they are used currently.
The press briefing will take place on Saturday, December 11, at 8:30 a.m. Eastern time in press briefing room A315.
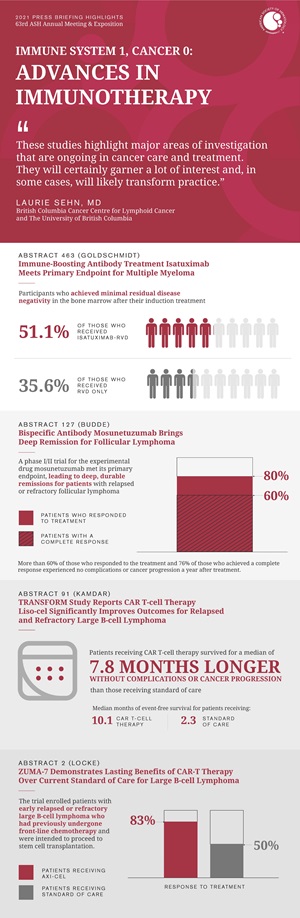
Immune-Boosting Antibody Treatment Isatuximab Meets Primary Endpoint for Multiple Myeloma
463: Addition of Isatuximab to Lenalidomide, Bortezomib and Dexamethasone as Induction Therapy for Newly-Diagnosed, Transplant-Eligible Multiple Myeloma Patients: The Phase III GMMG-HD7 Trial
Patients receiving the anti-CD38 monoclonal antibody isatuximab in addition to the standard three-component induction therapy lenalidomide, bortezomib, and dexamethasone (RVd) for newly diagnosed multiple myeloma were significantly more likely to achieve minimal residual disease negativity (no evidence of cancer in the bone marrow) compared with those receiving RVd alone, according to results from the first primary endpoint of a phase III trial.
About half (50.1%) of study participants who received isatuximab-RVd versus 35.6% of those receiving RVd only achieved minimal residual disease negativity in the bone marrow after their induction treatment.
“This is the first phase III trial to successfully challenge a standard of care that is broadly used in the U.S. and Europe,” said Prof. Hartmut Goldschmidt, MD, of the Heidelberg University Hospital (UKHD) and National Center of Tumor Diseases Heidelberg (NCT) in Germany. “Our results support this treatment as a new standard of care in transplant-eligible patients with newly diagnosed myeloma.”
Treatment for multiple myeloma, a cancer of the plasma cells that is most common in people over the age of 60, has advanced substantially in recent years, achieving a 10-year survival rate of up to 70%. By adding isatuximab to RVd, the researchers aimed to further increase survival rates and completely eradicate the cancer in the bone marrow for more patients. Isatuximab is approved in the U.S., Europe, and other countries for multiple myeloma patients who have already undergone previous rounds of treatment. This new trial assessed its use as part of the first-line treatment for newly diagnosed, transplant-eligible patients up to 70 years of age.
“Isatuximab acts in two ways – one is the direct effect of the antibody on myeloma cells, and the other is the immunostimulatory effect,” said Dr. Goldschmidt. “The idea is that if the immune system is stimulated by isatuximab, treatment of myeloma will be more effective.”
The trial enrolled 662 newly diagnosed patients at 67 medical centers throughout Germany. Half received induction therapy isatuximab plus RVd and half received RVd alone. The duration of induction therapy was 18 weeks for both treatment arms. In addition to meeting the trial’s primary endpoint for minimal residual disease negativity in the bone marrow, those receiving isatuximab were significantly more likely to achieve a response characterized as a very good partial response or better (a category that includes a complete response or a very good partial response) and less likely to show evidence of disease progression. However, there was no significant difference between arms in terms of the rate of complete response. The researchers also found no differences among subgroups, suggesting all patients benefit from the addition of isatuximab to RVd. There was no major difference between groups in terms of overall adverse events or serious adverse events. The most common adverse events in both groups were blood and lymphatic system disorders, infections, and nervous system disorders, with low white blood cell counts being more frequent in the isatuximab-RVd group.
The trial is continuing and will next assess the impact of isatuximab-RVd versus RVd induction therapy after autologous stem cell transplantation, as well as the drug’s potential effects when used as part of the maintenance regimen with lenalidomide.
Hartmut Goldschmidt, Heidelberg University Hospital (UKHD) and National Center of Tumor Diseases (NCT), will present this study during an oral presentation on Sunday, December 12, at 12:00 noon Eastern time in Hall A411-A412.
Bispecific Antibody Mosunetuzumab Brings Deep Remission for Follicular Lymphoma
127: Mosunetuzumab Monotherapy Is an Effective and Well-Tolerated Treatment Option for Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Who Have Received ≥2 Prior Lines of Therapy: Pivotal Results from a Phase I/II Study
A phase I/II trial for the experimental drug mosunetuzumab met its primary endpoint, leading to deep, durable remissions for patients with relapsed or refractory follicular lymphoma. In the single-arm study, 80% of patients responded to the treatment and 60% experienced a complete response.
“This is a very efficacious, very safe drug, even for subgroups of patients who typically do not respond well to existing therapies,” said L. Elizabeth Budde, MD, PhD, of City of Hope Comprehensive Cancer Center in California. “The results are a strong endorsement of the unique mechanism of action for this drug.”
Mosunetuzumab is a type of bispecific antibody designed to recognize a target on the lymphoma cells and a target on the patient’s own immune T cells and bring the two together. “It acts as a bridge to give a patient’s T cells guidance to help them get in the proximity of the lymphoma cell, become active, and destroy the lymphoma cell,” said Dr. Budde.
Unlike CAR T-cell therapy, which also uses T cells to fight cancer, mosunetuzumab can be infused directly into the bloodstream without requiring the removal and modification of patients’ immune cells.
The trial enrolled 90 patients who had seen their follicular lymphoma return after two or more rounds of treatment with existing therapies. Patients were infused with mosunetuzumab and followed for a median of more than 18 months. More than 60% of those who responded to the treatment and 76% of those who achieved a complete response experienced no complications or cancer progression a year after treatment.
Similar to CAR T-cell treatment, the trial also saw cytokine release syndrome (CRS), which occurred in 44.4% of patients. All but two instances of CRS were low-grade, and all CRS events were manageable and reversible. CRS is an inflammatory response common in immune-modulating therapies. Overall, the therapy was well tolerated and the participants, including older patients, did not require hospitalization. Many participants were able to continue their normal daily routines throughout treatment, researchers said.
“The goal is not only to treat the lymphoma but to improve the quality of life for patients,” said Dr. Budde. “By motivating our immune system to recognize lymphoma cells, this drug could give us an opportunity for chemotherapy-free treatment for follicular lymphoma.”
L. Elizabeth Budde, City of Hope, will present this study during an oral presentation on Saturday, December 11, at 12:00 noon Eastern time in Thomas Murphy Ballroom 1-2.
TRANSFORM Study Reports CAR T-cell Therapy Liso-cel Significantly Improves Outcomes for Relapsed and Refractory Large B-cell Lymphoma
91: Lisocabtagene Maraleucel (liso-cel), a CD19-Directed Chimeric Antigen Receptor (CAR) T Cell Therapy, Versus Standard of Care (SOC) with Salvage Chemotherapy (CT) Followed By Autologous Stem Cell Transplantation (ASCT) As Second-Line (2L) Treatment in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Results from the Randomized Phase 3 Transform Study
An interim analysis of the TRANSFORM trial comparing CAR T-cell therapy lisocabtagene maraleucel (liso-cel) to standard of care found that liso-cel significantly improved event-free survival for patients with large B-cell lymphoma that persisted or returned within 12 months after treatment with first line chemotherapy. Standard of care for this patient group consists of salvage chemotherapy and, for responding patients, additional high intensity chemotherapy followed by stem cell transplantation. The trial met its primary endpoint in that the patients receiving CAR T-cell therapy survived for a median of 10.1 months without complications or cancer progression, a substantial improvement over the median of 2.3 months of event-free survival among those receiving standard of care.
“The current standard of care consisting of chemotherapy and transplant is not effective at curing most patients with high risk relapsed large B-cell lymphoma, representing a huge unmet need in our field,” said Manali Kamdar, MD, of the University of Colorado Cancer Center. The FDA has currently approved liso-cel as a third-line treatment for lymphoma patients whose cancer doesn’t respond to two prior lines of therapy. The TRANSFORM study was designed to assess its efficacy against standard of care as a second-line treatment, potentially making patients eligible for CAR T-cell therapy sooner and avoiding the need to go through stem cell transplantation.
“Despite a relatively short follow-up period of just over six months, the positive results of this study suggest that CAR T-cell therapy has the potential to become the new standard of care for patients who do not respond to initial chemotherapy or who relapse within 12 months,” said Dr. Kamdar.
Researchers randomized 184 patients to receive liso-cel or standard of care. All participants had relapsed or refractory large B-cell lymphoma and were eligible to receive a stem cell transplant. In addition to its demonstrated superiority in terms of the trial’s primary endpoint of event-free survival, liso-cel was found to significantly extend the median length of survival without disease progression by nine months compared to standard of care. Liso-cel also increased the likelihood of achieving a complete response to treatment, which occurred in 66% of those receiving liso-cel and only 39% of those receiving standard of care. Of 92 patients randomized to receive standard of care, 50 patients ultimately crossed over to receive liso-cel.
The safety profile of liso-cel was comparable to the standard of care and some patients were able to receive liso-cel infusion in the outpatient clinic setting. CAR T-cell therapy can cause side effects such as CRS and neurological toxicity. Although roughly half of patients receiving liso-cel experienced CRS and 12% experienced neurological toxicity (with the most common neurological side effects being headaches, dizziness, tremors, and problems with speech), these CAR T-cell therapy related toxicities were low grade and reversible, with no grade 4 or 5 CRS or neurological events. There were no deaths attributable to liso-cel treatment.
“This is a breakthrough therapy which has shown superiority over standard of care in terms of efficacy with an extremely favorable safety profile. We are excited about the potential of this study to change the existing standard of care in these high-risk patients,” said Dr. Kamdar.
The researchers will continue to follow patients to assess any differences in overall survival at the time of primary analysis.
Manali Kamdar, University of Colorado Cancer Center, will present this study during an oral presentation on Saturday, December 11, at 9:30 a.m. Eastern time in Hall A1.
ZUMA-7 Demonstrates Lasting Benefits of CAR-T Therapy Over Current Standard of Care for Large B-cell Lymphoma
2: Primary Analysis of ZUMA‐7: A Phase 3 Randomized Trial of Axicabtagene Ciloleucel (Axi-Cel) Versus Standard‐of‐Care Therapy in Patients with Relapsed/Refractory Large B-Cell Lymphoma
Two-year data from the ZUMA-7 trial primary analysis show that the CAR T-cell therapy axicabtagene ciloleucel (axi-cel) significantly improved event-free survival compared to standard of care for patients with aggressive large B-cell lymphoma, meeting the trial’s primary endpoint. With a median of over two years of follow-up, patients receiving axi-cel survived without needing additional cancer treatment or experiencing cancer progression for a median of 8.3 months while those receiving standard of care had a median event-free survival of just two months. Overall, 41% of those receiving axi-cel and 16% of those receiving standard of care survived for two years without needing additional cancer treatment or experiencing cancer progression.
The standard of care second-line treatment for this patient group consists of additional chemotherapy; if the lymphoma responds to this additional chemotherapy, patients can then be eligible to undergo high dose chemotherapy and hematopoietic stem cell transplantation. Axi-cel is currently approved by the U.S. Food and Drug Administration (FDA) as a third-line therapy for large B-cell lymphoma; the ZUMA-7 trial assessed its use as a second-line therapy.
“The results of ZUMA-7 herald a paradigm shift in how we treat large B-cell lymphoma,” said Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa. “We found that by giving axi-cel in the second line setting, patients had longer event-free survival compared to the standard of care. This is remarkable and indicates that patients with lymphoma not responding to initial treatment or relapsing within 12 months should have the opportunity to get this therapy.”
The trial enrolled 359 patients with early relapsed or refractory large B-cell lymphoma who had previously undergone front-line chemotherapy and were intended to proceed to stem cell transplantation. Although the data for overall survival are not yet mature, those receiving axi-cel had a higher rate of event-free survival (as described above) and a higher rate of response to treatment, which occurred in 83% of patients receiving axi-cel and 50% of patients receiving standard of care.
The rates of adverse events were relatively similar between both study arms, with adverse events of grade 3 or higher occurring in 91% of patients receiving axi-cel and 83% of those receiving standard of care. The most common event in both groups was cytopenias, or low blood cell counts. Previous studies have found CAR T-cell therapy leads to two types of acute toxicities, which are usually transient: CRS and neurologic events. In this trial, 6% of patients receiving axi-cel experienced CRS of grade 3 or higher, and 21% experienced neurologic events of grade 3 or higher, with 12% experiencing changes in brain function (encephalopathy), which were temporary in most cases. Among those receiving standard of care, 27% experienced fever when their white blood cell counts were low due to chemotherapy, which is considered a serious event. “For both study arms, the rates and types of adverse events were consistent with expectations based on previous trials and real-world experience,” Dr. Locke noted.
“By giving CAR T-cell therapy as an earlier line of treatment, we are able to reduce the amount of chemotherapy patients are exposed to and get them quickly to a definitive therapy that can eradicate lymphoma for many years, if not forever, without a stem cell transplant,” said Dr. Locke.
The trial did not include cross-over between study arms but allowed progressing patients to receive any subsequent anti-cancer therapy including CAR T-cell therapy as a standard of care for third line or later treatment. Ultimately, 56% of those randomized to receive standard of care received CAR T-cell therapy after their cancer progressed.
Researchers will continue to follow patients for survival and analyze outcomes among subgroups of patients.
Frederick L. Locke, Moffitt Cancer Center, will present this study during a plenary presentation on Sunday, December 12, at 2:00 p.m. Eastern time in Hall B211-B212.
Additional press briefings will take place throughout the meeting on diversifying care in acute leukemia, new insights into blood disorders, COVID-19, and selected late-breaking abstracts. For the complete annual meeting program and abstracts, visit www.hematology.org/annual-meeting. Follow ASH and #ASH21 on Twitter, Instagram, LinkedIn, and Facebook for the most up-to-date information about the 2021 ASH Annual Meeting.
The American Society of Hematology (ASH) (www.hematology.org) is the world’s largest professional society of hematologists dedicated to furthering the understanding, diagnosis, treatment, and prevention of disorders affecting the blood. For more than 60 years, the Society has led the development of hematology as a discipline by promoting research, patient care, education, training, and advocacy in hematology. ASH publishes Blood (www.bloodjournal.org), the most cited peer-reviewed publication in the field, and Blood Advances (www.bloodadvances.org), an online, peer-reviewed open-access journal.
Contact:
Leah Enser, American Society of Hematology
[email protected], 202-552-4927