Studies Aim to Improve Outlook for Those Living with Sickle Cell Disease
New insights on health care utilization and potential treatments
(Orlando, FL, Dec. 8, 2019) — Three studies being presented today during the 61st American Society of Hematology (ASH) Annual Meeting and Exposition in Orlando highlight efforts to offer better treatments and more comprehensive care for people living with sickle cell disease (SCD).
“These studies together illustrate how progress is being made all the way from the laboratory bench to dissemination and implementation of better treatments and systems-level quality improvements,” said press briefing moderator Julie Panepinto, MD, Medical College of Wisconsin. “Researchers are moving the needle with studies both here in the U.S. and around the world.”
The first study reveals a protein’s role in repressing fetal hemoglobin and suggests a potential lead for new drugs that would help people living with SCD make healthy red blood cells. The second study, conducted in Africa, offers compelling evidence that oral arginine supplements could enhance pain relief during SCD-related episodes of severe pain known as vaso-occlusive crises and reduce associated hospitalizations. The third documents a fragmentation of SCD care during the transition from adolescence to early adulthood, drawing attention to factors that may undermine the delivery of consistent, coordinated care.
Supporting SCD research and access to care is a chief priority for ASH, which in 2016 launched a multifaceted initiative to address the burden of this disease both in the United States and globally. As a part of the initiative, ASH developed clinical guidelines for SCD management and care, expanded education and training efforts, advocated with policymakers to enhance and expand federal SCD programs, and founded the Sickle Cell Disease Coalition. In addition to these efforts, the ASH Research Collaborative (ASH RC) SCD Clinical Trials Network was developed with the mission to improve outcomes for individuals with SCD by expediting SCD therapy development and facilitating innovation in clinical trial research.
This press conference will take place on Sunday, December 8, at 8 a.m. in the ASH press briefing room W221DE.
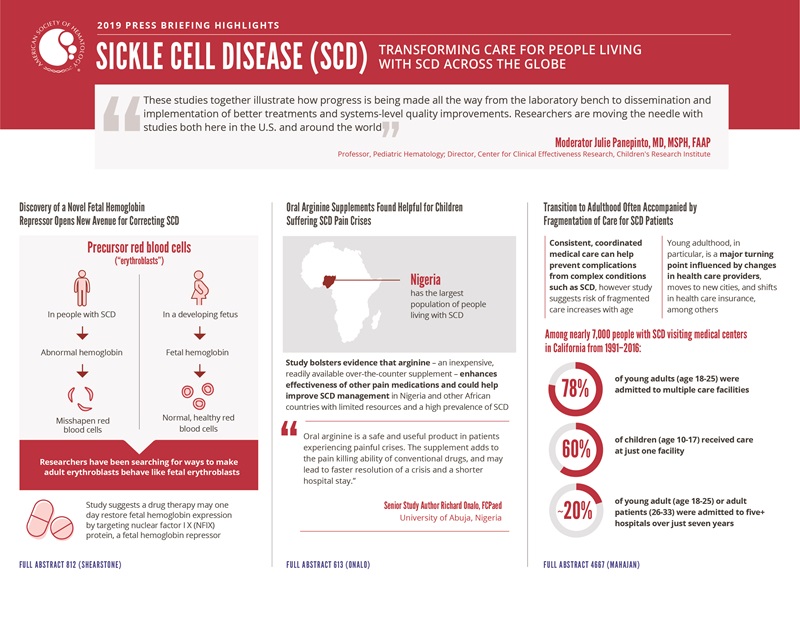
Discovery of a Novel Fetal Hemoglobin Repressor Opens New Avenue for Correcting SCD
#812 Chromatin Accessibility Mapping of Primary Erythroid Cell Populations Leads to Identification and Validation of Nuclear Factor I X (NFIX) as a Novel Fetal Hemoglobin (HbF) Repressor
A new study suggests a protein known as nuclear factor I X (NFIX) could offer an opportunity to correct the red blood cell abnormalities that are at the heart of SCD. The research confirms NFIX’s role in helping precursor red blood cells switch from fetal hemoglobin expression to adult hemoglobin expression. This finding suggests NFIX could one day be targeted by a drug for SCD to restore fetal hemoglobin expression, allowing the body to make healthy red blood cells instead of sickle-shaped ones.
In people living with SCD, precursor red blood cells known as erythroblasts produce faulty hemoglobin, causing red blood cells to become misshapen. However, erythroblasts in a developing fetus produce a slightly different form of hemoglobin, known as fetal hemoglobin, which makes normal, healthy red blood cells. Researchers have been searching for ways to make adult erythroblasts behave like fetal erythroblasts in order to help people with SCD produce more normal red blood cells.
In the new study, researchers from Syros Pharmaceuticals studied gene activity and hemoglobin production at discrete stages of development in fetal and adult erythroblasts. They traced how proteins known as transcription factors work to turn on, or shut off, genes related to fetal and adult hemoglobin production. Following up on results from their analysis and hints from previous studies indicating NFIX may play a role, they discovered that NFIX acts as a transcription factor that prevents adult erythroblasts from making fetal hemoglobin.
“Having a new transcription factor on the table as a fetal hemoglobin repressor opens up a potential new avenue for drug discovery,” said senior study author Jeffrey R. Shearstone, PhD, of Syros. “There’s still a lot to learn, but identification of a new fetal hemoglobin repressor and understanding how it impacts gene regulatory networks within erythroblasts gets us a little closer to being able to develop new therapies.”
To confirm NFIX’s role in repressing fetal hemoglobin, the team decreased the expression of NFIX in adult erythroblasts. They found that without NFIX, the cells produced fetal hemoglobin. It is not clear whether NFIX directly represses fetal hemoglobin production or if this process is mediated indirectly, through regulation of other genes and proteins. This research is part of Syros’ broader effort to develop drugs to increase the expression of fetal hemoglobin in people with SCD, and future studies are aimed at understanding the mechanisms by which NFIX and other genes silence fetal hemoglobin to elucidate potential therapeutic targets, Dr. Shearstone said.
Mudit Chaand, PhD, Syros Pharmaceuticals, will present this study during an oral presentation on Monday, December 9, at 4:30 p.m. in Valencia A (W415A).
Oral Arginine Supplements Found Helpful for Children Suffering SCD Pain Crises
#613 Oral Arginine Therapy as a Novel Adjuvant in the Management of Acute Pain in Children with Sickle Cell Anemia in Nigeria: A Randomized Placebo-Controlled Trial
Children who were given oral arginine supplements during vaso-occlusive crises as a result of SCD reported lower levels of pain, required less pain medication, and were discharged from the hospital earlier than those given a placebo, on average, in a clinical trial conducted in Nigeria.
Nigeria has a larger population of people with SCD than any other country. The study bolsters the evidence that arginine enhances the effectiveness of other pain medications and suggests arginine could help improve SCD management in Nigeria and other African countries with limited resources and a high prevalence of SCD, according to researchers.
“Arginine is a cheap, readily available over-the-counter supplement,” said senior study author Richard Onalo, FCPaed, University of Abuja, Nigeria. “Oral arginine is a safe and useful product in patients experiencing painful crises. The supplement adds to the pain killing ability of conventional drugs, and may lead to faster resolution of a crisis and a shorter hospital stay.”
Arginine, an amino acid, is a key building block for nitric oxide, a bio-regulatory molecule that relaxes blood vessels. During a vaso-occlusive crisis, small blood vessels in affected parts of the body become obstructed, depriving tissues of oxygen. At the same time, concentrations of arginine drop precipitously, limiting the body’s ability to produce nitric oxide and reopen the obstructed blood vessels.
Only a few previous studies have investigated the use of arginine supplements to replenish depleted arginine and help resolve vaso-occlusive crises. One U.S. trial found children given intravenous arginine when hospitalized for SCD-related pain had a significant decrease in pain and opioid use compared to placebo. The new study is the first conducted in Africa and the first to test the use of arginine supplements delivered orally, rather than through an injection, for acute pain control, Dr. Onalo said.
The researchers enrolled 68 children hospitalized for vaso-occlusive crises at two hospitals in Abuja, Nigeria. Half were given oral arginine every eight hours until hospital discharge or up to 15 doses total, while half were given a placebo on the same schedule.
Children given arginine had their pain decline more quickly, reached the end of their vaso-occlusive crisis faster, and required less total pain medication overall, though the decrease in total opioid dose was not statistically significant. More than half of children receiving arginine were discharged from the hospital by day five while only a quarter of children receiving placebo were discharged by that point.
The researchers observed no difference between the groups in terms of serious adverse events, though children receiving arginine were more likely to experience vomiting. The arginine supplement is unpalatable, Dr. Onalo noted, though the taste typically can be masked by mixing the supplement with Gatorade or grape juice.
Taken together, the new study and the previous trial of intravenous arginine in the U.S. make a strong case for further research, Dr. Onalo said. For example, future studies could help to determine whether giving oral arginine supplements at home at the onset of crisis or in the hospital for those already on admission could prevent hospitalizations and readmissions in children with SCD-related pains. Additionally, future studies could examine the use of oral arginine supplementation in adults with SCD and assess how disease severity, race, and location affect the benefits of arginine for managing vaso-occlusive crises.
Richard Onalo, FCPaed, University of Abuja, Nigeria will present this study during an oral presentation on Monday, December 9, at 10:30 a.m. in W304ABCD.
Transition to Adulthood Often Accompanied by Fragmentation of Care for SCD Patients
#4667 Fragmentation of Care for Young Adults with SCD in California
Consistent, coordinated medical care can help prevent complications from complex conditions such as SCD. However, a new study found that most young adults with SCD – 78% – were admitted to multiple care facilities, fragmenting their medical care. By contrast, more than half –60% – of children with SCD received care at just one facility, suggesting fragmentation begins during the transition from adolescence to early adulthood.
“There are more people who are working to bring children into the health care system, whereas when people get older it becomes something they have to manage for themselves,” said senior study author Anjlee Mahajan, MD, of the University of California, Davis School of Medicine. “Young adulthood is often a period of transition; they might move to a new town or city and there are often shifts in health insurance. That point is when their care gets fragmented.”
The researchers analyzed health records of nearly 7,000 people with SCD who visited medical centers in California between 1991-2016. They divided patients into three age groups each covering a seven-year span from age 10-17 (childhood), 18-25 (young adulthood) and 26-33 (adulthood). Roughly one in five young adult or adult patients were admitted to five or more hospitals over just seven years.
The data revealed a stark shift in which patients went from receiving care at one or two facilities in childhood to over half of patients receiving care at three or more facilities after age 18. This fragmentation did not resolve as patients reached their late 20s and early 30s. Patients lacking health insurance, those visiting the hospital more frequently, and those who did not always receive care at specialized SCD centers tended to have the most fragmented care.
Based on the study, it is unclear how fragmentation affects patient outcomes, Dr. Mahajan noted. Patients who were more frequently admitted to the hospital overall faced the highest risk of death. About a quarter of young adult patients were admitted 30 or more times over a seven-year time period, an average of more than four hospitalizations per year. While fragmentation of care was associated with a higher frequency of visits, care fragmentation was not independently associated with increased risk of death.
Given that SCD requires active management to prevent pain crises and long-term organ damage, Dr. Mahajan suggested future studies could help identify the impacts of fragmentation and perhaps point to ways to support more consistent care throughout the transition to adulthood.
Ashley Shatola, MD, University of California, Davis, will present this study in a poster presentation on Saturday, December 7, at 5:30 p.m. in Hall B.
The study authors and press program moderator will be available for interviews after the press conference or by telephone. Additional press briefings will take place throughout the meeting on VTE, inclusive medicine, CAR-T and beyond, and late-breaking abstracts. For the complete annual meeting program and abstracts, visit www.hematology.org/annual-meeting. Follow @ASH_hematology and #ASH19 on Twitter and like ASH on Facebook for the most up-to-date information about the 2019 ASH Annual Meeting.
The American Society of Hematology (ASH) (www.hematology.org) is the world's largest professional society of hematologists dedicated to furthering the understanding, diagnosis, treatment, and prevention of disorders affecting the blood. For 60 years, the Society has led the development of hematology as a discipline by promoting research, patient care, education, training, and advocacy in hematology. ASH publishes Blood (www.bloodjournal.org), the most cited peer-reviewed publication in the field, which is available weekly in print and online. In 2016, ASH launched Blood Advances (www.bloodadvances.org), an online, peer-reviewed open-access journal.
Contacts:
Adam Silverstein, FleishmanHillard
917-697-9313; [email protected]
Leah Enser, ASH
202-552-4927; [email protected]