Major Trials Show Benefits of New Therapy Regimens for Cancer and Blood Disorders
Large-scale Phase III trials highlight key advances in lymphoma, leukemia, myelodysplastic syndromes, and beta thalassemia
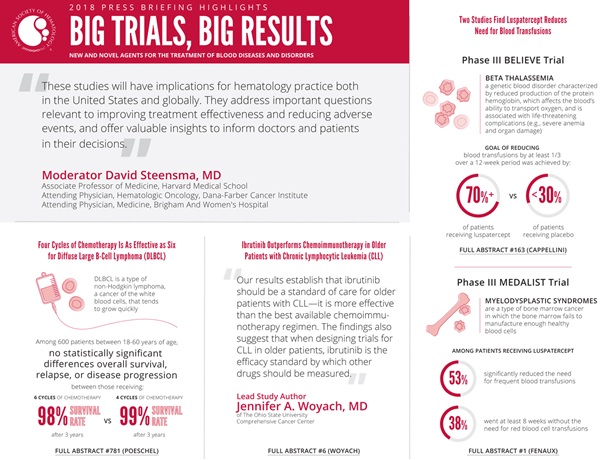
(San Diego, December 1, 2018) — Today during the 60th American Society of Hematology (ASH) Annual Meeting and Exposition in San Diego, researchers will present the results of four large trials that evaluate promising new treatment options for patients with several types of malignant and non-cancerous blood disorders.
“These studies will have implications for hematology practice both in the United States and globally,” said press briefing moderator David Steensma, MD, of Dana-Farber Cancer Institute and Harvard Medical School. “They address important questions relevant to improving treatment effectiveness and reducing adverse events and offer valuable insights to inform doctors and patients in their decisions.
One study reveals the benefits of reducing the number of cycles of chemotherapy for treating patients with diffuse large B-cell lymphoma. A second study compares several drug combinations for treating older patients with chronic lymphocytic leukemia.
A pair of separate studies suggest the experimental drug luspatercept can help improve red blood cell production, reducing the need for transfusions in patients with anemia arising from different causes. One study shows luspatercept was helpful for patients with beta thalassemia, while the other focuses on myelodysplastic syndromes.
This press conference will take place on Saturday, December 1, at 9:30 a.m. PST in Room 22, San Diego Convention Center.
Four Cycles of Chemotherapy as Effective as Six for Diffuse Large B-Cell Lymphoma
Excellent Outcome of Young Patients (18-60 years) with Favourable-Prognosis Diffuse Large B-Cell Lymphoma (DLBCL) Treated with 4 Cycles CHOP Plus 6 Applications of Rituximab: Results of the 592 Patients of the Flyer Trial of the Dshnhl/GLA [781]
A new study suggests the standard course of treatment for younger patients with low-risk DLBCL can safely be reduced by two cycles of chemotherapy. The trial, which tracked patients for a median of more than five years and up to 11 years, showed four cycles of chemotherapy is as effective as six cycles in terms of eradicating cancer and preventing relapse.
Researchers said the ability to reduce chemotherapy cycles by one-third is likely to come as welcome news for patients, who often must put their lives on hold as they cope with the side effects of chemotherapy. Under the reduced regimen, chemotherapy lasts a total of 84 days, compared to 126 days with the six-cycle regimen.
“With a shorter duration of chemotherapy, patients are back to daily life with their families and back to work more quickly,” said lead study author Viola Poeschel, MD, of Saarland University Medical School in Homburg/Saar, Germany. “Our study shows you can spare two cycles of chemotherapy and it is equally effective. We think this will be the new standard treatment for this patient population.”
DLBCL is a type of non-Hodgkin lymphoma, a cancer of the white blood cells, that tends to grow quickly. Most patients receive treatment in six cycles spaced three weeks apart. Each treatment cycle includes a chemotherapy regimen known as CHOP, which includes cyclophosphamide, doxorubicin, vincristine, and prednisone. Along with CHOP, patients receive rituximab, a monoclonal antibody drug that attacks cancerous cells via a different mechanism. Rituximab is less toxic, and therefore has fewer side effects compared to chemotherapy.
For the study, researchers enrolled nearly 600 patients age 18-60 treated for DLBCL at institutions in Germany, Denmark, Norway, Italy, and Israel. All patients had stage I or II cancer and were considered to be low-risk. Half of the patients were randomly assigned to receive six cycles of CHOP and half received four cycles of CHOP. All patients received the standard six cycles of rituximab.
Patients did well with both treatment regimens, and there were no statistically significant differences between the two groups in terms of overall survival, relapse, or disease progression. Three years after receiving treatment, 99 percent of those receiving four cycles of chemotherapy and 98 percent of those receiving six cycles remained alive.
In addition, the data suggest that reducing the number of chemotherapy cycles also reduces the number of adverse events by one-third. Altogether, 1,295 adverse events occurred in the 295 patients who underwent six cycles of chemotherapy compared with 835 adverse events in the 293 patients who received just four cycles of chemotherapy. “This is an important and meaningful benefit to patients,” Dr. Poeschel added.
The researchers will continue to track the health of study participants for an additional five years to determine whether decreasing the number of chemotherapy cycles may help reduce long-term side effects of chemotherapy.
This study was supported by Deutsche Krebshilfe, a German non-profit foundation.
Viola Poeschel, MD, Saarland University Medical School, will present this study during an oral presentation session on Monday, December 3, at 2:45 p.m. PST in Ballroom 20A, San Diego Convention Center.
Ibrutinib Outperforms Chemoimmunotherapy in Older Patients with Chronic Lymphocytic Leukemia
Ibrutinib Alone or in Combination with Rituximab Produces Superior Progression Free Survival (PFS) Compared with Bendamustine Plus Rituximab in Untreated Older Patients with Chronic Lymphocytic Leukemia (CLL): Results of Alliance North American Intergroup Study A041202 [6]
A new study shows older patients with chronic lymphocytic leukemia (CLL) have a significantly lower rate of disease progression if treated with the targeted therapy ibrutinib rather than the chemotherapy drug bendamustine plus the antibody rituximab — the combination regimen previously considered to be one of the most effective therapies for this group of patients. The study, which is the first head-to-head comparison between these treatment regimens, also suggests adding rituximab to ibrutinib does not add benefits beyond those seen with ibrutinib alone.
CLL is a cancer of the white blood cells that is most common in older adults. The U.S. Food and Drug Administration (FDA) approved ibrutinib as a first-line treatment for CLL in 2016.
“Our results establish that ibrutinib should be a standard of care for older patients with CLL — it is more effective than the best available chemoimmunotherapy regimen,” said lead study author Jennifer A. Woyach, MD, of The Ohio State University Comprehensive Cancer Center. “The findings also suggest that when designing trials for CLL in older patients, ibrutinib is the efficacy standard by which other drugs should be measured.”
The trial enrolled 547 older patients with previously untreated, symptomatic CLL between 2013 –2016. Participants ranged from 65-89 years old. One-third were randomly assigned to receive bendamustine plus rituximab, one-third received ibrutinib plus rituximab, and one-third received ibrutinib alone. Researchers tracked patient outcomes for a median of 38 months, just over three years.
Rates of survival without any disease progression at two years (the study’s primary endpoint) were significantly better in patients who received ibrutinib plus rituximab (88%) and ibrutinib alone (87%) compared with bendamustine plus rituximab (74%). However, there was no difference in overall survival among the three groups at two years. In this study, patients whose disease progressed after receiving bendamustine plus rituximab crossed over to receive ibrutinib as a second-line treatment.
The addition of rituximab to ibrutinib did not appear to improve outcomes compared with receiving ibrutinib alone. Overall, patients responded well to all three treatment regimens, with an overall response rate (tumor reduction) of 94 percent in those receiving ibrutinib alone, 93 percent in those receiving ibrutinib plus rituximab, and 81 percent in those receiving bendamustine plus rituximab.
As seen in previous trials, ibrutinib was associated with some significant side effects. In particular, up to 17 percent of patients receiving ibrutinib developed atrial fibrillation, an abnormal heart rhythm that increases the risk of strokes and other cardiovascular problems. The toxicities observed in the ibrutinib arms of this trial warrant further study and caution in using this drug in all older patients, Dr. Woyach noted.
Despite the fact that CLL is most common in older people, the trial is one of just a few that have included only patients over age 65. Most clinical trials for CLL have been conducted in younger adults. In addition, ibrutinib’s FDA approval for first-line treatment of CLL in 2016 was based on comparison to chemotherapy with chlorambucil, a drug approved in 1957 which is considered an obsolete therapy by most clinicians. The comparison of ibrutinib to bendamustine and rituximab is of much more interest, since the latter regimen is currently widely used for treatment of patients with CLL over age 60.
“The study highlights the importance of doing clinical trials for older patients, because the toxicities are likely to be different for older versus younger patients, even with the same drug,” said Woyach.
Jennifer A. Woyach, MD, The Ohio State University Comprehensive Cancer Center, will present this study during the plenary session on Sunday, December 2, at 2:00 p.m. PST, Hall AB, San Diego Convention Center.
Two Studies Suggest Luspatercept Reduces Need for Blood Transfusions
Separate phase III clinical trials show luspatercept to be safe and effective at reducing the need for blood transfusions. The studies focus on patients with beta thalassemia and myelodysplastic syndromes, respectively.
The BELIEVE Trial: Results of a Phase III, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept in Adult Beta-Thalassemia Patients Who Require Regular Red Blood Cell (RBC) Transfusions [163]
In a phase III clinical trial, the experimental drug luspatercept significantly reduced the need for blood transfusions in patients with the inherited blood disorder beta thalassemia. More than 70 percent of patients who received luspatercept were able to cut blood transfusions by at least one-third over any consecutive 12-week period, while just under 30 percent of those who received a placebo were able to achieve the same result.
Beta thalassemia is a genetic blood disorder characterized by reduced production of the protein hemoglobin, which affects the blood’s ability to transport oxygen, and is associated with life-threatening complications such as severe anemia and organ damage. Although new treatments have emerged in recent years, including curative gene therapies, blood transfusions remain the standard of care and most accessible treatment for a vast majority of patients. Many patients require transfusions every few weeks.
Luspatercept is designed to increase the amount of healthy red blood cells by interfering with signals that suppress red blood cell production, thus improving patients’ ability to manufacture their own red blood cells and reducing the need for transfusions.
“Beta thalassemia is a very demanding disease,” said lead study author Maria Domenica Cappellini, MD, of the University of Milan in Italy. “This new approach can totally change the quality of life for the patient. In addition, even for those who don’t become completely transfusion independent, reducing transfusions can reduce associated comorbidities.”
Receiving a blood transfusion carries a small risk of infection or adverse immune reaction. In addition, patients who require frequent, regular blood transfusions over the course of years often suffer liver and heart problems due to a buildup of iron.
The trial enrolled 336 adult patients with beta thalassemia. Participants were mostly young, with a median age of 30 years, and required a median of six units of blood over a 12-week period before the trial. Two-thirds were randomly assigned to receive luspatercept and one-third received a placebo, both administered via an injection every three weeks.
The researchers tracked the number of units of blood each participant required over the course of 48 weeks (about 11 months).
The results show those taking luspatercept were 5.8 times more likely to reach the primary endpoint (a 33% reduction in the number of units of blood needed during the study period) compared with those taking the placebo. By the final quarter of the trial, about 20 percent of patients overall had cut their number of transfusion units by one-third or more, and 10 percent of patients had cut their transfusion units by half or more.
Reported adverse events included bone pain and thrombotic events, such as stroke, but the rates of these events did not differ significantly between patients taking luspatercept and those taking the placebo.
Genetic variation is known to affect the severity of beta thalassemia, so it may also affect the efficacy of treatments. The researchers plan to further analyze the data to determine whether genes or other factors affect how patients respond to luspatercept.
Maria Domenica Cappellini, MD, University of Milan, will present this study during an oral presentation session on Saturday, December 1, at 2 p.m. PST, Room 30D, San Diego Convention Center.
The MEDALIST Trial: Results of a Phase 3, Randomized, Double-Blind, Placebo-Controlled Study of Luspatercept to Treat Anemia in Patients with Very Low-, Low-, or Intermediate-Risk Myelodysplastic Syndromes (MDS) with Ring Sideroblasts (RS) Who Require Red Blood Cell (RBC) Transfusions [1]
In a phase III clinical trial, the experimental drug luspatercept significantly reduced the need for frequent blood transfusions in just over half (53%) of patients with myelodysplastic syndromes (MDS) who were anemic, required regular red blood cell transfusions, and showed abnormal iron overload in red blood cell precursors (a condition known as ring sideroblasts) before the study. Thirty-eight percent of patients who received luspatercept were able to avoid red blood cell transfusions for at least eight weeks.
Myelodysplastic syndromes (MDS) are a type of bone marrow cancer in which the bone marrow fails to manufacture enough healthy blood cells. Patients with higher-risk forms of the disease often develop bleeding, infections, or acute myeloid leukemia, a more aggressive type of blood cancer. Among those with lower-risk MDS, anemia is the most common subtype of low blood count and causes debilitating symptoms for many patients.
Available anemia drugs only work for about one-half of patients with lower-risk MDS-related anemia and about one-quarter of those who are dependent on red blood cell transfusions. Patients who do not respond to these drugs or have adverse reactions to them may require frequent blood transfusions to boost their supply of red blood cells and hemoglobin, the oxygen-carrying component of blood.
Red blood cell transfusions increase hemoglobin only temporarily, and frequent transfusions are costly and time-consuming. They can also lead to iron overload, especially in the heart and liver.
“Anemia and the chronic need for transfusions is a very big issue for these patients,” said lead study author Pierre Fenaux, MD, PhD, of Hôpital Saint-Louis in Paris. “With low hemoglobin levels, patients are tired all the time and have an increased risk of falls and cardiovascular events. When you can improve hemoglobin levels, you really see a difference in quality of life.”
The trial enrolled 229 adult patients with low, very low, or intermediate-risk MDS with ring sideroblasts. All patients had either failed to respond to available anemia drugs or were ineligible for treatment with such drugs, and required red blood cell transfusions at least every one to two months. Two-thirds were randomly assigned to receive luspatercept and one-third received a placebo, both administered via an injection every three weeks for at least six months.
More than one-third (38%) of patients receiving luspatercept and 13 percent of those receiving a placebo achieved the primary endpoint of at least eight weeks without a need for a red blood cell transfusion. Twenty-eight percent of patients receiving luspatercept and 8 percent of patients receiving a placebo achieved the secondary endpoint of at least 12 weeks without transfusion. Overall, 53 percent of patients experienced either a significant reduction in the number of transfusions required or an increase in hemoglobin levels even without transfusions, compared with 12 percent of patients receiving placebo.
The most common reported adverse effects with luspatercept treatment included fatigue and muscle pain, though it is difficult to determine whether these effects were related to anemia or to the drug itself. It is also unclear whether the drug would offer benefits for patients with higher-risk MDS or those without lower-risk MDS without ring sideroblasts, researchers noted, since only low- and intermediate-risk patients with ring sideroblasts were included in the trial.
Alan F. List, MD, Moffitt Cancer Center, Tampa, FL, will present this study during the plenary presentation on Sunday, December 2, at 2:00 p.m. PST, Hall AB, San Diego Convention Center.
The study authors and press program moderator will be available for interviews after the press conference or by telephone. Additional press briefings will take place throughout the meeting on improved sickle cell disease outcomes, lasting results in CAR T-cell therapies, and looking to the future in the era of personalized medicine. For the complete annual meeting program and abstracts, visit http://www.hematology.org/annual-meeting. Follow @ASH_hematology and #ASH18 on Twitter and like ASH on Facebook for the most up-to-date information about the 2018 ASH Annual Meeting.
The American Society of Hematology (ASH) (www.hematology.org) is the world's largest professional society of hematologists dedicated to furthering the understanding, diagnosis, treatment, and prevention of disorders affecting the blood. For more than 50 years, the Society has led the development of hematology as a discipline by promoting research, patient care, education, training, and advocacy in hematology. The Society publishes Blood (www.bloodjournal.org), the most cited peer-reviewed publication in the field, as well as the newly launched, online, open-access journal, Blood Advances.
Contacts:
Adam Silverstein, FleishmanHillard
917-697-9313; [email protected]
Stephen Fitzmaurice, ASH
561-506-6890; [email protected]