CAR T-Cell Therapies Drive Outcomes in Lymphoma, Myeloma
Treatments Hold Promise for People with Hard-to-Treat Blood Cancers
(Atlanta, December 10, 2017) — For people with certain types of aggressive, refractory blood cancers, treatment options are woefully limited. But three studies being presented today at the 59th American Society of Hematology (ASH) Annual Meeting and Exposition in Atlanta spotlight the emerging role played by chimeric antigen receptor (CAR) T-cell therapies in helping individuals mount a clinical response and, in some cases, achieve durable remission.
These therapies are designed by harvesting a patient’s own T-cells (the immune system’s primary cancer-killing cells), reengineering them to target specific proteins on the surface of leukemia and lymphoma cells, and reintroducing the modified T-cells back into the patient’s immune system.
“It is encouraging that the data continue to be so strong and suggest that CAR-T therapies for B-cell malignancies are here to stay,” said press briefing moderator, Renier J. Brentjens, MD, PhD, medical oncologist and director of cellular therapeutics at Memorial Sloan Kettering Cancer Center. “There is still a lot we need to learn about toxicities — for example, how to manage cytokine release syndrome (CRS), a common, potentially dangerous reaction to this type of infusion.”
In two separate, longer-term follow-up analyses (of the ZUMA-1 and JULIET trials), researchers found that initial responses were sustained over time in patients who received genetically modified T cells designed to target the CD-19 protein, which is frequently expressed on malignant lymphoma cells. A third, Phase I study — one of the largest to evaluate a CAR therapy targeting BCMA, a marker present on the vast majority of multiple myeloma tumor cells — showed encouraging early results in patients with heavily pre-treated multiple myeloma.
“It’s an exciting time. Based on these results and recent FDA approvals in this field, there is reason to be confident that cell therapies, such as CAR-T, may one day be the standard of care for hematologic malignancies as well as solid tumors,” said Dr. Brentjens.
This press conference will take place on Sunday, December 10, at 10:30 a.m. EST in Room A315 of the Georgia World Congress Center.
Responses to CAR T-Cell Therapy Still Strong after One Year in Patients with Refractory NHL, Data also Shed Light on Why Therapy Fails in Some Patients
Long-Term Follow-up ZUMA-1: A Pivotal Trial of Axicabtagene Ciloleucel (Axi-Cel; KTE-C19) in Patients with Refractory Aggressive Non-Hodgkin Lymphoma (NHL) [578]
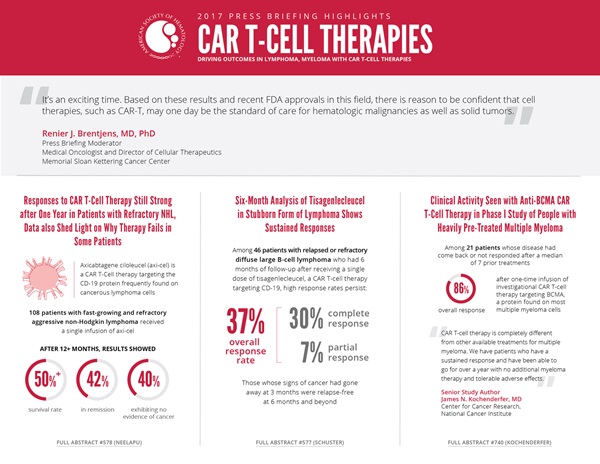
Among 108 patients with fast-growing and refractory aggressive non-Hodgkin lymphoma (NHL), more than half were still alive at least a year after receiving a single infusion of a CAR T-cell therapy, axicabtagene ciloleucel (axi-cel), that targets the CD-19 protein frequently found on cancerous lymphoma cells, researchers reported. This latest analysis of ZUMA-1, which combines Phase I and II trial data, assessed the rate and durability of responses and survival among these patients after a median follow-up of 15.4 months. More than one year after a single infusion of axi-cel, 42 percent of patients remain in remission and 40 percent of patients exhibit no evidence of cancer.
“Long-term follow-up of ZUMA-1 confirms that these responses can be durable and the ongoing responses at 24 months suggest that late relapses are uncommon. Patients who are in remission at 6 months tend to stay in remission,” said lead study author Sattva S. Neelapu, MD, professor at The University of Texas MD Anderson Cancer Center. “With existing therapy, the median survival for people with this disease is only 6 months. Here, we see more than half of patients — 59 percent — are still alive over a year after treatment.”
The study, which is being conducted at 22 sites, is the largest study of a CAR T-cell therapy’s efficacy to date, according to researchers. Dr. Neelapu explains that the durability findings are also consistent with observations from earlier, single-institution trials of axi-cel in this patient population. In terms of safety, no new deaths related to the therapy occurred. Early in the study, four patients died within two months of treatment — two attributable to the CAR T-cell therapy and the other two to unrelated adverse side effects that are typical of disease progression. In the pivotal portion of ZUMA-1, common adverse events consisted of CRS, neurologic toxicities, neutropenia, anemia, and thrombocytopenia. Ten patients experienced a serious adverse event six months after the primary analysis, including infections in eight patients. No new onset CRS or neurologic events related to axi-cel were observed in the updated analysis.
The study also provides some of the first clues as to why some patients relapse or do not respond to CAR T-cell therapy After analyzing tumor tissue from before and after treatment in patients who relapsed, the researchers found that in a third of patients the CD19 protein was no longer present on cancer cells. Secondly, more than two-thirds of tumors showed evidence of another protein, PD-L1, likely helping the cancer cells survive by inhibiting the function of the infused T cells. Follow-up studies are now underway to identify possible approaches to overcoming these problems.
There are roughly 72,000 new cases of NHL in the U.S. each year. NHL starts in white blood cells called lymphocytes, which are part of the immune system. There are two main types of lymphocytes — B-cells and T-cells — whose role is to help the body fight infection.
A randomized trial to compare the efficacy of this therapy with second-line standard of care, which includes autologous stem cell transplantation for relapse after first-line therapy, is planned in patients with aggressive B-cell NHL.
Funding for this study was provided by Kite Pharma, Inc., now Gilead Sciences.
Sattva S. Neelapu, MD, The University of Texas MD Anderson Cancer Center, will present this study during an oral presentation on Monday, December 11, at 7:00 a.m. EST in Room A411 of the Georgia World Congress Center.
Six-Month Analysis of Tisagenlecleucel in Stubborn Form of Lymphoma Shows Sustained Responses
Primary Analysis of Juliet: A Global Pivotal Phase 2 Trial of CTL019 in Adult Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma [577]
Six months after receiving a single dose of tisagenlecleucel, a CAR T-cell therapy that targets CD-19, high response rates persist among adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
This latest interim analysis of the international JULIET trial showed that for 46 patients with at least 6 months of follow-up, the overall response rate was 37 percent, with 30 percent achieving a complete response and 7 percent achieving a partial response. What’s more, according to researchers, this observation indicates that, among 81 patients treated, those whose signs of cancer had gone away at 3 months remained relapse-free at 6 months and beyond.
“While we don’t completely understand why these remissions are so durable, it’s exciting and will change how this disease is treated when conventional therapies fail,” said lead study author Stephen Schuster, MD, Professor of Hematology/Oncology in the Perelman School of Medicine at the University of Pennsylvania (Penn) and Penn's Abramson Cancer Center. “We are going to be able to offer patients who don’t respond to standard therapies a form of therapy that may, after a single treatment, relieve symptoms and save lives.”
DLBCL is the most common form of lymphoma, accounting for roughly one-third of all non-Hodgkin lymphoma cases. While current therapy is successful for many people with this disease, those not responding to current treatments face a poor prognosis with limited treatment options. According to Dr. Schuster, primary therapy will fail in about one-third of people with DLBCL, and half of these patients will not be candidates for stem cell transplantation, which is considered the best second-line treatment approach; such patients would be candidates for this type of therapy.
This single-arm, open-label Phase II trial is the largest study examining a CAR T-cell therapy exclusively in people with DLBCL. It is being conducted at 27 sites spanning 10 countries across North America, Europe, Australia, and Asia. Enrollees had received two or more lines of prior chemotherapy and had disease progression, or had failed to respond or were ineligible for autologous stem cell transplant. Patients ranged in age from 22 to 76 years old.
Subgroup analyses showed no difference in outcomes based on prior DLBCL treatment or risk factors. Of the 81 patients included in JULIET, the responding patients continue to be followed without any additional therapy, and median durable overall response and overall survival have yet to be reached.
Most of the adverse events were seen shortly after infusion and included CRS and neurotoxicities. There were no deaths attributable to CTL019, CRS, or neurological events.
Dr. Schuster said several factors set this trial apart from other investigations of CAR T-cell therapies, including that the therapy was done on an outpatient basis for many patients (26 percent) and the manufacturing process allowed investigators to generate CAR T cells from previously collected and frozen blood cells, permitting successful shipment around the world.
“Once the CAR T cells were generated, we could freeze them again, allowing us to hold the product until patients were clinically ready to receive them,” he said. “These are very sick patients, so this gives the treating physician some flexibility to schedule therapy when it’s best for each patient.”
Patients in the JULIET trial who responded to therapy continue to be followed carefully for recurrence of their lymphoma and recovery of their immune system.
Funding for this study was provided by Novartis.
Stephen J. Schuster, MD, University of Pennsylvania, will present this study during an oral presentation on Monday, December 11, at 7:00 a.m. in Room A411 of the Georgia World Congress Center.
Clinical Activity Seen with Anti-BCMA CAR T-Cell Therapy in Phase 1 Study of People with Heavily Pre-Treated Multiple Myeloma
Durable Clinical Responses in Heavily Pretreated Patients with Relapsed/Refractory Multiple Myeloma: Updated Results from a Multicenter Study of bb2121 Anti-BCMA CAR-T Cell Therapy [740]
A one-time infusion of an investigational CAR T-cell therapy that targets a protein found on most multiple myeloma cells elicited an 86-percent overall response rate in 21 patients whose disease had come back or had not responded after a median of seven prior treatments, according to results from a Phase I study.
Among 18 patients who received higher, active doses of infused CAR T cells, this response rate increased to 94 percent, with manageable adverse effects, researchers reported. Among these 18 patients, 10 achieved a complete response and 9 of 10 evaluated for minimal residual disease (MRD) using sensitive genetic tests achieved an MRD-negative response. After a median follow-up period of 40 weeks, the median progression-free survival had not been reached; four patients who received active doses had seen their disease get worse.
“We are excited about the early results in a patient population with very advanced myeloma for whom previous therapies have failed,” said senior study author James N. Kochenderfer, MD, of the Center for Cancer Research at the National Cancer Institute in Bethesda, Maryland.
These findings are important, Dr. Kochenderfer said, because despite recent therapeutic advances, multiple myeloma — a cancer that begins in plasma cells, cells in the bone marrow that help the body fight infection — remains nearly incurable. Existing therapies require patients to stay on treatment long-term with drugs that have side effects, he said.
“CAR T-cell therapy is completely different from other available treatments for multiple myeloma,” Dr. Kochenderfer said. “We have patients who have a sustained response and have been able to go for over a year with no additional myeloma therapy and tolerable adverse effects.”
The study, conducted at nine sites in the United States, is the first U.S.-based multicenter study of a CAR T-cell therapy engineered to target BCMA, a protein found on the vast majority of both myeloma tumor cells and normal plasma cells, but no other healthy tissues. An estimated 30,000 people in the United States will be diagnosed with multiple myeloma in 2017.
Twenty-one patients with a median age of 58 years were enrolled in the dose-escalation phase of the study. All had seen their disease come back or stop responding after a median of seven prior treatments, including a stem cell transplant.
The primary objective of the Phase I study was to identify the “maximum tolerated dose” of the experimental treatment — that is, the highest dose that could be given without unacceptable levels of adverse effects. Additional outcome measures included evaluating whether any cancer cells remained in the bone marrow, the length of time until the cancer began to get worse, and response to treatment as measured by a standard set of criteria for assessing multiple myeloma.
Most patients experienced adverse effects, including low blood counts, CRS, and neurologic symptoms. All three patients treated at an inactive CAR-T dose, the lowest dose in the dose-escalation stage of the trial, died from progression of their myeloma within one year. Among the 18 patients treated at active CAR-T doses, two patients died from other causes while their myeloma was in a complete response to CAR-T therapy.
These findings are preliminary and, as a Phase I trial, the study had no control group and was designed primarily to identify a safe dose of bb2121, not to evaluate the drug’s effectiveness.
Funding for this multi-site study was provided by Celgene Corporation and bluebird bio, Inc.
James N. Kochenderfer, MD, National Cancer Institute, will present this study during an oral presentation on Monday, December 11, at 2:45 p.m. EST in Hall C1 of the Georgia World Congress Center.
The study authors and press program moderator will be available for interviews after the press conference or by telephone. Additional press briefings will take place throughout the meeting. For the complete annual meeting program and abstracts, visit www.hematology.org/annual-meeting. Follow @ASH_hematology and #ASH17 on Twitter and like ASH on Facebook for the most up-to-date information about the 2017 ASH Annual Meeting.
The American Society of Hematology (www.hematology.org) is the world’s largest professional society of hematologists dedicated to furthering the understanding, diagnosis, treatment, and prevention of disorders affecting the blood. For more than 50 years, the Society has led the development of hematology as a discipline by promoting research, patient care, education, training, and advocacy in hematology. ASH publishes Blood (www.bloodjournal.org), the most cited peer-reviewed publication in the field, which is available weekly in print and online. In 2016, ASH launched Blood Advances (www.bloodadvances.org), an online, peer-reviewed open-access journal.
CONTACTS:
Stephen Fitzmaurice, ASH
561-506-6890; [email protected]
Adam Silverstein, FleishmanHillard
917-697-9313; [email protected]