Prevention of COVID-19 in Immunocompromised Patients With Hematologic Conditions
While we regularly review and update the information provided here, this FAQ reflects only the best information available at a given point in time. The COVID-19 pandemic is a rapidly evolving global health crisis and new information may have become available since the revision date for each published FAQ.
Which patients are considered moderately or severely immunocompromised?
To generate optimal protective immunity following vaccination, intact host immunity is needed, particularly with respect to antigen presentation, B- and T-cell activation, and plasma B-cell antibody generation. Therefore, hosts lacking functional adaptive immune cells may be unable to generate a fully protective immune response to a SARS-CoV-2 vaccine approved for use in the general population.
The following immunocompromised patient populations could have attenuated or absent response to SARS-CoV-2 vaccines:
- Primary and secondary immunodeficiencies involving adaptive immunity
- Splenectomy or functional asplenia
- B-cell–directed therapies (e.g., blocking monoclonal antibodies against CD20 or CD22, bispecific agents such as blinatumomab, CD19- or CD22-directed chimeric antigen receptor T-cell [CAR-T] therapies, or Bruton tyrosine kinase [BTK] inhibitors)
- T-cell–directed therapies (e.g., calcineurin inhibitors, antithymocyte globulin, alemtuzumab)
- Many chemotherapy regimens
- High-dose corticosteroids (>2 mg/kg/d daily prednisone > 2 weeks, or equivalent)
- Hematopoietic cell transplantation (HCT), especially within the first three to six months after autologous HCT, and often longer after allogeneic HCT
- Underlying aberrant immunity (e.g., graft-vs.-host disease, graft rejection, absent or incomplete immune reconstitution, neutropenia ANC <500/μL, lymphopenia ALC <200/μL)
There are currently no recommendations from the Centers for Disease Control and Prevention (CDC) for routinely monitoring response to vaccination in immunocompromised patients. The reader is referred to the American Society for Transplantation and Cellular Therapy for additional guidance for patients who have undergone HCT or Chimeric Antigen T-cell receptor therapy.
What are the currently available vaccines against coronavirus available in the United States?
In the United States, there are four approved or authorized vaccines to prevent COVID-19 or reduce severity of infection.1,2 Pfizer-BioNTech and Moderna are COVID-19 mRNA vaccines. There is also a protein subunit vaccine made by Novavax that contains COVID-19 spike protein subunits and an anjuvant.3 In some situations, the Johnson & Johnson/Janssen COVID-19 viral vector vaccine may be available.4 The BNT162b2 (Pfizer/BioNTech) and the mRNA-1273 (Moderna) COVID-19 vaccines have both been shown in large phase III clinical trials to be more than 90 percent effective at preventing lab-confirmed COVID-19 illness and severe infections. The single-dose recombinant, replication-incompetent adenovirus serotype 26 vector-based vaccine (Ad26.COV2.S; Johnson & Johnson/Janssen) reduced the incidence of symptomatic COVID-19 with a reported overall efficacy of 66.1 percent (72% in the United States) based on data from the phase III clinical trial.
Is vaccination effective in immunocompromised patients?
The early clinical trials on novel vaccines excluded patients with most hematologic conditions. However, since approval, multiple studies have been performed and published estimating the effectiveness of coronavirus vaccine strategies in a variety of immunosuppressed populations.5-8 There is a high level of heterogeneity in the severity of immunosuppression and the response to the vaccines depending on disease, therapy, and multiple patient factors, making application to specific immunosuppressed populations challenging, though a growing number of meta-analyses have provided some guidance.9-12
Additionally, there are thus far no data relevant to the use of individualized testing of antibody response after vaccination to predict efficacy.
In the absence of a robust understanding of vaccine response and disease risk, the CDC has recommended a vaccination schedule specifically for individuals who fall into the moderately or severely immunocompromised category.
What is the schedule of vaccination and booster for patients who are immunocompromised?
The CDC’s recommendations for vaccination are currently based on which initial vaccination was provided. They are available on the CDC website (COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised; Use of COVID-19 Vaccines in the United States).
Figure: COVID-19 Vaccination Schedule for People Who Are Moderately or Severely Immunocompromised
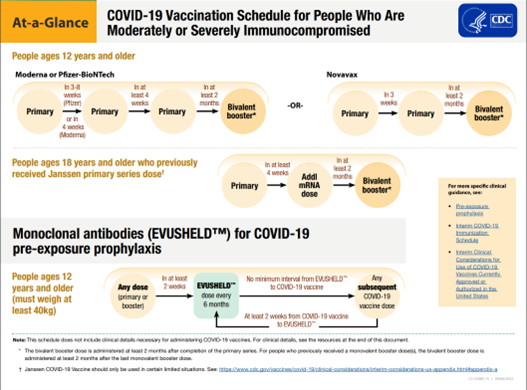
*Adapted from https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
Should I use serological testing to decide if my patient should receive the third vaccine dose?
Serological testing before the third dose is not recommended at this time. The currently available assays are nonstandardized, and the clinical thresholds of protection are not known. Third doses and boosters are administered regardless of serological results or antibody levels if the patient meets eligibility criteria.
Should immunocompromised patients be treated prior to infection with monoclonal antibodies?
Currently, no monoclonal antibodies are available in the United States either via FDA approval or Emergency Use Authorization (EUA) to treat immunocompromised patients either prior to infection, following exposure, or with documented infection, due to the lack of predicted efficacy against the omicron family of SARS-CoV-2 variants currently circulating in the United States and the world.
References
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384(5):403-416. doi: 10.1056/NEJMoa2035389.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615. doi: 10.1056/NEJMoa2034577.
- Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385(13):1172-1183. doi: 10.1056/NEJMoa2107659.
- Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384(23):2187-2201. doi: 10.1056/NEJMoa2101544.
- Haydu JE, Maron JS, Redd RA, et al. Humoral and cellular immunogenicity of SARS-CoV-2 vaccines in chronic lymphocytic leukemia: a prospective cohort study. Blood Adv. 2022;6(6):1671-1683. doi: 10.1182/bloodadvances.2021006627.
- Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, et al. Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv. 2022;6(5):1537-1546. doi: 10.1182/bloodadvances.2021006917.
- Herishanu Y, Rahav G, Levi S, et al. Efficacy of a third BNT162b2 mRNA COVID-19 vaccine dose in patients with CLL who failed standard 2-dose vaccination. Blood 2022;139(5):678-685. doi: 10.1182/blood.2021014085.
- Marasco V, Carniti C, Guidetti A, et al. T-cell immune response after mRNA SARS-CoV-2 vaccines is frequently detected also in the absence of seroconversion in patients with lymphoid malignancies. Br J Haematol. 2022;196(3):548-558. doi: 10.1111/bjh.17877.
- Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10(3):e326-e328. doi: 10.1016/s2214-109x(21)00593-3.
- Noori M, Azizi S, Abbasi Varaki F, et al. A systematic review and meta-analysis of immune response against first and second doses of SARS-CoV-2 vaccines in adult patients with hematological malignancies. Int Immunopharmacol. 2022;110:109046. doi: 10.1016/j.intimp.2022.109046.
- Mehrabi Nejad MM, Shobeiri P, Dehghanbanadaki H, et al. Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: a systematic review and meta-analysis. Virol J. 2022;19(1):132. doi: 10.1186/s12985-022-01858-3.
- Lee A, Wong SY, Chai LYA, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632. doi: 10.1136/bmj-2021-068632.
- Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of COVID-19. N Engl J Med. 2022;386(23):2188-2200. doi: 10.1056/NEJMoa2116620.