Case Study: 78-Year-Old Woman with Thrombocytopenia and Splenomegaly
A 78-year-old woman with a history of hypertension and hypothyroidism presented with thrombocytopenia with a platelet count of 60,000/mm3, as well as a hemoglobin of 13.2 g/dL and white blood cell count of 4,300/µL. Moderate splenomegaly was noted on physical examination. Review of her peripheral smear demonstrated rare platelets with normal platelet morphology. The patient was started on intravenous steroids for a working diagnosis of immune thrombocytopenia (ITP). A computed tomography (CT) scan of the abdomen demonstrated an enlarged spleen measuring 19.5 × 15 cm (Figure 1a). She underwent a bone marrow biopsy (Figure 1b) that demonstrated a population of cells with the following immunophenotype on flow cytometry: CD19+, CD20+, surface IgM+, CD23-, CD5-, CD10-, CD11c-, and CD103-.
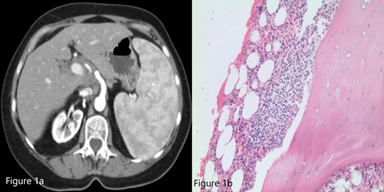
Figure 1a. CT scan of abdomen demonstrating significant splenomegaly (19.5 × 15 × 10 cm) with inhomogeneous parenchymal enhancement.
Figure 1b. Bone marrow biopsy (40×, Jenner-Giemsa stain).
What is the most likely diagnosis?
- Extranodal marginal zone lymphoma
- Splenic marginal zone lymphoma
- Follicular lymphoma
- Diffuse large B-cell lymphoma
Answer: B
ITP in patients older than 60 years with splenomegaly should raise suspicion for chronic lymphocytic leukemia or lymphoma.1,2 The bone marrow biopsy showed a sinusoidal pattern of infiltration, consistent with marginal zone lymphoma (MZL), specifically splenic MZL given prominent splenomegaly. The patient was initially treated with six cycles of single-agent rituximab infusion from 2010 to 2011, with subsequent remission. Post-treatment abdominal imaging demonstrated a significant decrease in the size of the spleen, from 19.5 cm to 13 cm. In 2014, she presented with relapsed disease in her bilateral parotid glands; a CT of the head and neck demonstrated multiple hyperdense structures within the enlarged parotid glands most consistent with intraparotid lymph nodes. Biopsy of an intraparotid lymph node demonstrated involvement of MZL (Figures 2 and 3). She subsequently received single-agent rituximab for six cycles followed by two years of maintenance therapy. In 2017, the patient had an asymmetrical enlargement of the right lacrimal gland (Figure 4). She underwent orbitectomy and complete resection of the mass, with pathology consistent with extranodal MZL. The immunophenotypic findings were CD19+, CD20+, CD38+, CD45+, CD5-, CD10-, CD23-, CD11c-, and CD103-. Postoperative MRI of the right orbit showed no evidence of the previously seen lacrimal mass. CT of the body did not show any lymphadenopathy or extranodal masses. Repeat bone marrow biopsy did not reveal involvement with MZL. Two years later, in 2019, the patient developed left temporal swelling, and an MRI of the face showed enhancement in the left temporal region. She denied biopsy of the mass but agreed to treatment, and she received four cycles of chemotherapy with R-CHOP (rituximab, cyclophosphamide, vincristine, and prednisone) with complete resolution of the mass on follow-up imaging. The patient remains in remission to date.
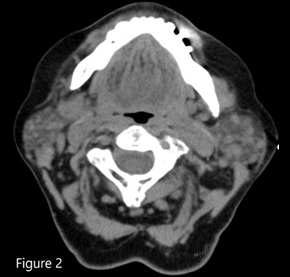
Figure 2. CT head and neck showing multiple hyperdense lesions in the parotid gland consistent with intraparotid lymph nodes.
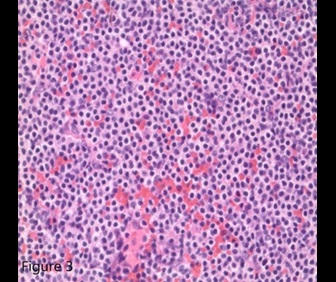
Figure 3. Lymph node biopsy consistent with marginal zone lymphoma (40×, Jenner-Giemsa stain).
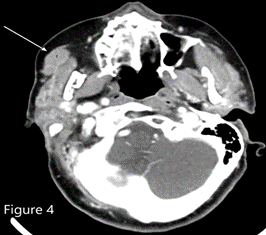
Figure 4. CT of the head and neck showed asymmetric enlargement of the right lacrimal gland (most prominently the palpebral lobe as marked by the white arrow).
We report an atypical case of MZL that illustrates the need for longitudinal follow-up due to an increased risk of relapse, including relapse outside of the initial presumed site of disease. MZL is a type of non-Hodgkin lymphoma (NHL) that arises from the marginal zone B-cells that are present in nodal and extranodal tissues. It can be further categorized into three different variants, depending on the focus of origin: splenic marginal zone lymphoma (SMZL), extranodal mucosa-associated lymphoid tissue (MALT) lymphoma, and nodal marginal zone lymphoma (NMZL).3 SMZL is the second most common type of MZL, representing 1 to 2 percent of all NHL cases and originating from the B lymphocytes that are present in the marginal zone of the spleen.4 It often presents with splenomegaly, as in our case, and is differentiated from nodal and extranodal MZL by immunohistochemistry as shown in Table 1. SMZL in its early stages is usually asymptomatic at presentation, with incidental findings of anemia, thrombocytopenia, or lymphocytosis, while advanced stages present as splenomegaly, abdominal pain, and early satiety.5
Table 2 presents differences in the epidemiology and presentations of various MZL subtypes. Although SMZL is a low-grade lymphoma, it can transform to an aggressive histology; therefore, biopsy at the time of progression at new sites may be necessary to exclude transformed lymphoma. Bone marrow and peripheral blood histology with immunophenotyping is commonly used because while the gold standard for diagnosis is splenic histology, this can often be difficult to obtain.6,7 The peripheral blood histology in SMZL shows villous lymphocytes described as round nuclei, condensed chromatin, and basophilic cytoplasm with polar short villi.7 As SMZL is a low-grade and indolent NHL, treatment is not always offered. Indications for treatment include symptomatic splenomegaly and progressive cytopenias. Management of SMZL also requires testing for hepatitis C infection, as antiviral therapy can result in regression of the lymphoma. Initial treatment is usually rituximab alone or in conjunction with splenectomy, though some studies suggest that splenectomy alone is not as effective as rituximab monotherapy for inducing remission.8 Further, splenectomy does not treat SMZL outside of the spleen, including the bone marrow involvement in the case of our patient. Relapsed disease is treated with combinations of rituximab and chemotherapy, such as R-CHOP or rituximab and bendamustine.5
Table 1. Immunohistochemistry features differences of SMZL, EMZL, NMZL and other B-cell lymphomas.
| SMZL | LPL | SDRPL | ENMZL/NMZL | MCL | FL | |
| CD20 | + | + | + | + | + | + |
| CD79a | + | + | + | + | + | + |
| CD5 | -/+ | -/+ | -/+ | -/+ | + | - |
| CD21 | -/+ | - | - | - | - | - |
| CD23 | -/+ | -/+ | - | -/+ | - | -/+ |
| BCL1 | - | - | - | - | + | - |
| DBA44 | +/- | - | + | - | - | - |
| CD103 | - | - | - | - | - | - |
| IRTA1 | - | - | - | +/- | - | - |
| IgM | + | + | + | + | + | + |
| IgD | +/- | - | -/+ | -/+ | + | + |
| CD10 | - | - | - | - | - | +/- |
| BCL6 | - | - | - | -/+ | -/+ | + |
| CD43 | -/+ | - | - | -/+ | + | - |
| SOX11 | - | - | - | - | + | - |
| LEF1 | - | - | - | - | -/+ | - |
Legend: -, <25% of cases; -/+, 25-50% of cases; +/-, 50-75% of cases; +, >75% of cases Abbreviations: SMZL, splenic marginal zone lymphoma; LPL, lymphoplasmacytic lymphoma; SDRPL, splenic diffuse red pulp lymphoma; NMZL, nodal marginal zone lymphoma; ENMZL, extranodal marginal zone lymphoma; MCL, mantle cell lymphoma; FL, follicular lymphoma. Table adapted from Arcaini L, et al. Blood. 2016;127:2072-2081.
Table 2. Characteristic differences among different types of MZL.
| Characteristics |
SMZL | NMZL | EMZL |
| Median age at diagnosis (y) | 69 | 50-60 | 50-60 |
| Association | Hepatitis C | Hepatitis C | Hepatitis C, Helicobacter pylori, Campylobacter jejuni, Borrelia burgdorferi |
| Presentation | Anemia, thrombocytopenia, splenomegaly |
Non-bulky abdomen, thoracic and peripheral lymph nodal involvement. | Extranodal involvement |
| Bone marrow involvement | Common, have sinusoidal infiltration pattern | Seen in one third of patients | Rare |
| Peripheral blood involvement | Common, have villous lymphocytes. | Rare | Rare |
Abbreviations: SMZL, splenic marginal zone lymphoma; NMZL, nodal marginal zone lymphoma; ENMZL, extranodal marginal zone lymphoma. Table adapted from Denlinger NM, et al. Cancer Manag Res. 2018;10:615-624.
References
- Liu L, Wang H, Chen Y, et al. Splenic marginal zone lymphoma: a population-based study on the 2001-2008 incidence and survival in the United States. Leuk Lymphoma. 2013;54:1380-1386.
- Thieblemont C, Felman P, Berger F, et al. Treatment of splenic marginal zone B-cell lymphoma: an analysis of 81 patients. Clin Lymphoma. 2002;3:41-47.
- Joshi M, Sheikh H, Abbi K, et al. Marginal zone lymphoma: old, new, targeted, and epigenetic therapies. Ther Adv Hematol. 2012;3:275-290.
- Zhang S, Xuan Z, Zhang L, et al. Splenic marginal zone lymphoma: a case report and literature review. World J Surg Oncol. 2020;18:259.
- Denlinger NM, Epperla N, William BM. Management of relapsed/refractory marginal zone lymphoma: focus on ibrutinib. Cancer Manag Res. 2018;10:615-624.
- Thieblemont C, Davi F, Noguera ME, et al. Splenic marginal zone lymphoma: current knowledge and future directions. Oncology (Willston Park). 2012;26:194-202.
- Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127:2072-2081.
- Kalpadakis C, Pangalis GA, Angelopoulou MK, et al. Treatment of splenic marginal zone lymphoma with rituximab monotherapy: progress report and comparison with splenectomy. Oncologist. 2013;18:190-197.
Note: Figures 1 through 4 are from the Pathology Lab at Saint Joseph Mercy Oakland Hospital in Pontiac, Michigan.
Case contributed by Drs. Jasmeet Kaur, Emad Wahashi, and Sarah Fatima, Department of Internal Medicine, Saint Joseph Mercy Oakland Hospital, Pontiac, MI; and Dr. Mehrvaan Kaur, Department of Radiology, Saint Joseph Mercy Oakland Hospital, Pontiac, MI.