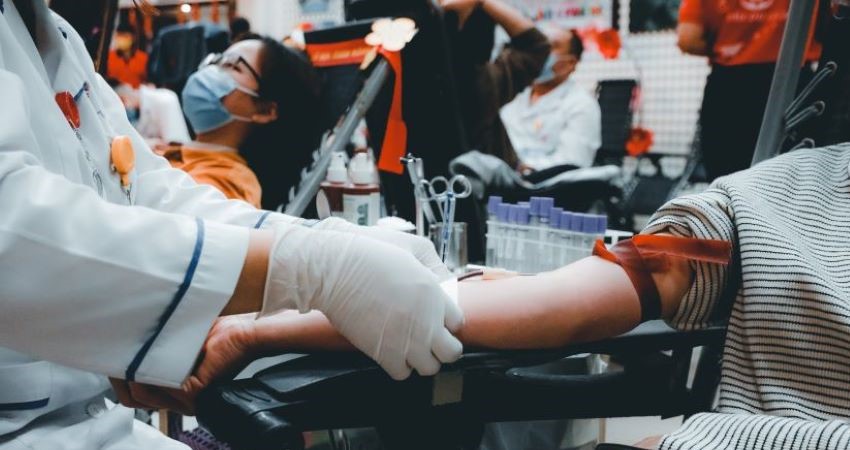
Join ASH in our relentless pursuit of change, as we actively shape policies that impact the very heart of hematology research and practice. We empower researchers, clinicians, and patients through education, equipping lawmakers, and government officials with the knowledge they need to make a difference. Together, we unite our voice to revolutionize the future of hematology for the better.
ASH Advocacy's Latest Impact on Hematology

FEBURARY 2024
Contact Congress in Support of Stopping Medicare Payment Cuts in 2024
Congress Seeks to Finish FY 2024 Funding with Start of FY 2025 Budget Process on Horizon
CMMI Launches Cell & Gene Therapy Access Model
CMS Finalizes Policies on Prior Authorization Nearly a Year After Proposed Rule was Released
ASH Joins Partners in Urging Supreme Court to Preserve FDA Authority

Research and Public Health Funding
ASH and other members of the biomedical research and public health communities continue to strongly urge Congress to provide increased annual funding for NIH, CDC and other federal public health agencies and programs-increased resources are needed now more than ever.
Access to Palliative Blood Transfusions
Misconceptions about transfusions and limited funds hinder access to patients' end-of-life care. ASH prompts calls for innovative reimbursement models and increased support for palliative transfusions in hospice settings.
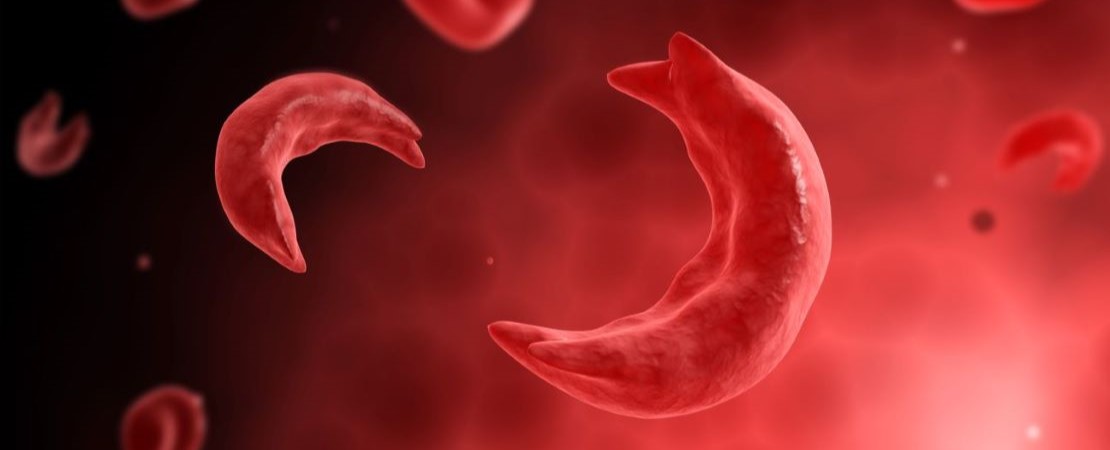
Sickle Cell Disease Advocacy
Since 2015, ASH has been committed to improving the outcomes for individuals living with SCD through collaborations with federal agencies, the U.S. Congress, and the U.S. Department of Health and Human Services.

Maternal Health Care
High maternal mortality rates in the United States, with a particular disparity among non-Hispanic Black women, underscore the need to address hematologic complications in pregnancy. While advocating for diverse maternal perspectives and experiences, ASH stands that no woman should face legal ramifications for childbearing decisions.
Policy News
Discover the most recent updates and insights in ASH Advocacy articles and news, ensuring you're up-to-date on the latest policy developments.
Policy Statements
Explore ASH's authoritative statements on various aspects of hematology, providing valuable guidance on research, practice, training, and the quality of care within the field.
Testimony and Correspondence
View current and formal congressional testimony and correspondence related to issues affecting hematologists and their patients.

The ASH Congressional Fellowship is a year-long opportunity that allows an ASH member to work in a congressional office on Capitol Hill, Washington, DC, for an academic year to help shape health care and hematology policy.

Advocacy Leadership Institute (ALI)
ALI is a two-day workshop in Washington, DC where ASH members can gain a better understanding of the Society, learn about how Congress and health policy impact hematology research and practice, and meet with Members of Congress and their staff on Capitol Hill.

ASH-FDA Collaboration
This Collaboration is a two-day workshop in Washington, DC, co-hosted by the U.S. Food and Drug Administration, for ASH members to network with FDA staff and learn about clinical and translational research, drug development, and regulatory medicine.