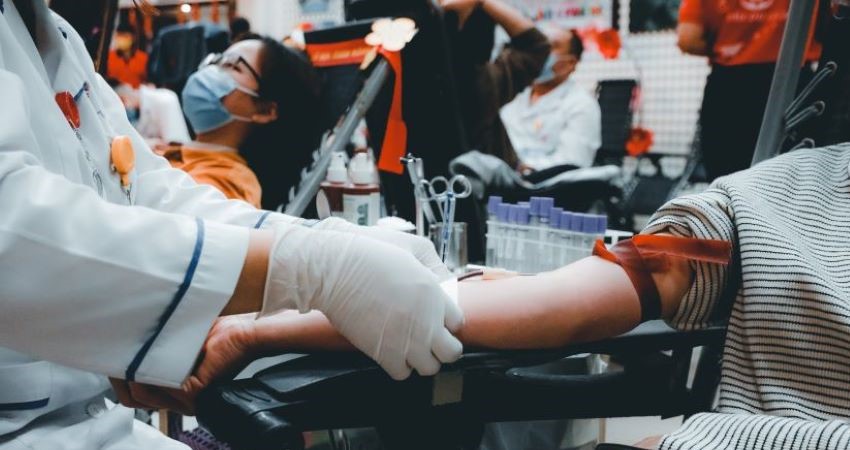
Join ASH in our relentless pursuit of change, as we actively shape policies that impact the very heart of hematology research and practice. We empower researchers, clinicians, and patients through education, equipping lawmakers, and government officials with the knowledge they need to make a difference. Together, we unite our voice to revolutionize the future of hematology for the better.
ASH Advocacy's Latest Impact on Hematology
Take ASH’s NEW Survey on NIH Funding Cuts and Delays
Take ASH’s survey to share how the Administration’s policies have impacted your research. Since the Administration took office, policies have been implemented that delay or restrict the release of appropriated funding for peer-reviewed grants, and we need to hear about how these changes are impacting you to inform ASH’s advocacy with the Administration and Congress.
ASH Survey on NIH Funding Cuts and DelaysSupport of Federal Research Funding
ASH continues to lead advocacy on behalf of hematologists in Washington as the new Administration issues executive orders and Congress moves to begin the process of drafting fiscal year (FY) 2026 spending bills, including federal research funding. ASH is calling on you to help support this critical advocacy.
Take Action in Support of Federal Research Funding
Advocacy News Roundup
Recapping Top Stories from June 2025
Trump Administration’s Full Budget Request Seeks to Slash NIH Funding
ASH Sends Letter to Senate Leadership to Reject Potential Cuts to Medicaid
ASH-Supported Legislation to Enhance Access to Palliative Blood Transfusions Reintroduced in Senate
Potential Changes to Medicare Physician Fee Schedule in House-Passed Reconciliation Package
ASH Submits Comments to CMS on FY 2026 Inpatient Prospective Payment System Proposed Rule
Federal Rule Summaries
See summaries of the latest rules on hematology-related topics.Policy Statements
Explore ASH's statements regarding research, practice, training, and quality of care within the field.Testimony and Correspondence
View formal congressional testimony and correspondence related to issues affecting hematologists and their patients.#Fight4Hematology
As the world’s largest professional society serving clinicians and scientists around the world who are working to conquer blood diseases, ASH is fighting for hematology in the face of executive orders and other federal actions that threaten hematology research and, ultimately, the care of patients.

Research and Public Health Funding
ASH and other members of the biomedical research and public health communities continue to strongly urge Congress to provide increased annual funding for NIH, CDC and other federal public health agencies and programs-increased resources are needed now more than ever.
Access to Palliative Blood Transfusions
Misconceptions about transfusions and limited funds hinder access to patients' end-of-life care. ASH prompts calls for innovative reimbursement models and increased support for palliative transfusions in hospice settings.
Sickle Cell Disease Advocacy
Since 2015, ASH has been committed to improving the outcomes for individuals living with SCD through collaborations with federal agencies, the U.S. Congress, and the U.S. Department of Health and Human Services.

Maternal Health Care
High maternal mortality rates in the United States, with a particular disparity among non-Hispanic Black women, underscore the need to address hematologic complications in pregnancy. While advocating for diverse maternal perspectives and experiences, ASH stands that no woman should face legal ramifications for childbearing decisions.

The ASH Congressional Fellowship is a year-long opportunity that allows an ASH member to work in a congressional office on Capitol Hill, Washington, DC, for an academic year to help shape health care and hematology policy.

Advocacy Leadership Institute (ALI)
ALI is a two-day workshop in Washington, DC where ASH members can gain a better understanding of the Society, learn about how Congress and health policy impact hematology research and practice, and meet with Members of Congress and their staff on Capitol Hill.

ASH-FDA Collaboration
This Collaboration is a two-day workshop in Washington, DC, co-hosted by the U.S. Food and Drug Administration, for ASH members to network with FDA staff and learn about clinical and translational research, drug development, and regulatory medicine.